PR News WireThis post was originally published on this site
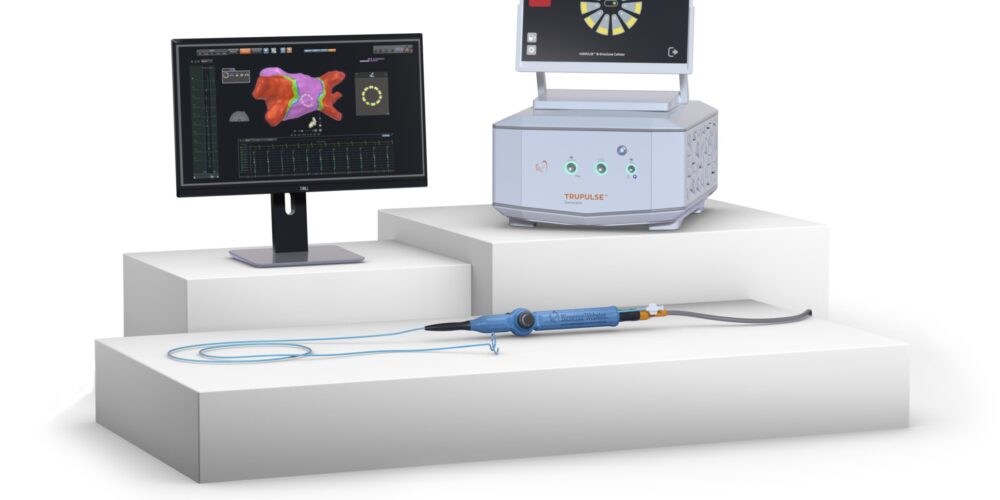
The VARIPULSE™ Platform is the first and only Pulsed Field Ablation (PFA) System in the U.S. fully integrated with the CARTO™ 3 Electro-anatomical Mapping System, driving efficiency, reproducibility and accuracy1
Approval is supported by the admIRE study, where 85% peak primary effectiveness 12-month success and minimal-to-no fluoroscopy were achieved2,i
IRVINE, Calif., Nov. 7, 2024 /PRNewswire/ — Johnson & Johnson MedTech, a global leader in cardiac arrhythmia treatment, today announced the U.S. Food & Drug Administration (FDA) approval of the VARIPULSE™ Platform for the treatment of drug refractory paroxysmal Atrial Fibrillation (AFib).
The VARIPULSE™ Platform is designed to enable AFib treatment with a single device that combines PFA therapy and advanced mapping with the CARTO™ 3 System, the world’s leading 3D electroanatomical cardiac mapping system. Strategically developed for full integration with CARTO™, the VARIPULSE™ Platform enables:
- The accuracy and safety of ablation procedures through precise energy delivery and real-time visualization of catheter positioning2,ii
- Minimal- to zero-fluoro workflow through seamless integration with the intracardiac echocardiography (ICE) ultrasound portfolio providing real-time imaging3,4,5
- Confidence in treatment delivered through tissue proximity indication and lesion tagging, providing electrophysiologists with feedback that has proven to be critical for lesion durability and long-term outcomes
- A single transseptal zero exchange workflow for an efficient and predictable procedure
- A comprehensive solution to seamlessly address both routine and complex AFib ablations2,iii
“We have learned that with PFA technology, mapping integration is critically important for electrophysiologists to ‘see’ inside the heart and deliver pulsed field energy with accuracy,” explained Luigi Di Biase, MD, PhD, FACC, FHRS, System Director Electrophysiology at Montefiore Health System, Professor of Medicine (Cardiology) Albert Einstein College of Medicine at Montefiore Hospital.iv “With today’s approval, electrophysiologists will have the ability to use an integrated mapping system – CARTO – for PFA procedures, enabling a singular, versatile workflow, that could reduce procedure time, potentially driving positive results for patients.”
The approval is supported by data from the admIRE study, a prospective, multi-center, non-randomized trial. Twelve-month outcome data from the pivotal phase of the admIRE study were published in Circulation. Among 291 patients across 30 healthcare centers in the U.S., 100% achieved acute procedural success, including 98% with first-pass isolation recorded per vein.2 85% achieved peak primary effectiveness when 73-96 applications were applied per vein (n=85), showed minimal adverse events (2.9%), and 25% of procedures were performed without fluoroscopy2, likely attributable to integration with the CARTO™ 3 System.2
“With this approval, we are excited to bring the VARIPULSE™ Platform to electrophysiologists and patients in the U.S., where AFib impacts nearly eight million people,” said Jasmina Brooks, President, Electrophysiology, Johnson & Johnson MedTech.6 “As the only PFA platform uniquely designed for seamless integration with the CARTO™ 3 System, we are confident that this eagerly awaited platform will be a valuable tool for physicians in performing safe, effective and efficient AFib procedures with an intuitive and reproducible workflow, and minimal-to-no fluoroscopy.”
AFib is the most common type of cardiac arrhythmia and affects more than 8 million people in the United States and more than 50 million people worldwide.6 Approximately 1 in 4 adults over the age of 40 are at risk for developing AFib.7 Despite these projections, about one-third of patients with AFib are not aware they have the condition, and AFib often goes unrecognized until the onset of complications.8,9 Catheter ablation is a safe and effective procedure when drugs don’t work to help restore the heart’s incorrect electrical signals, which cause an abnormal heart rhythm.10
“With a growing prevalence of atrial fibrillation around the world, innovative solutions are critical in expanding options for patients and helping electrophysiologists treat AFib effectively and efficiently,” said Andrea Natale, M.D., Executive Medical Director, Texas Cardiac Arrythmia Institute, St. David’s Medical Center.v “The VARIPULSE Platform enables efficient procedures with a favorable safety profile, allowing me to treat more patients and get them back to their normal lives.”
In addition to the VARIPULSE™ Platform, Johnson & Johnson MedTech is committed to developing a comprehensive suite of PFA technologies, including the investigational Dual Energy THERMOCOOL SMARTOUCH ™ SF Catheter – which is being studied to deliver both radiofrequency and PF energy, and the OMNYPULSE™ Catheter – a large-tip, 12 mm focal catheter with contact force sensing and a TRUEref™ reference electrode. Learn more about the J&J MedTech innovation in PFA here. The Dual Energy THERMOCOOL SMARTOUCH ™ SF Catheter and the OMNYPULSE™ Catheter are investigational and not available for sale or distribution in any market.
About the VARIPULSE™ Platform
The VARIPULSE™ Platform is Johnson & Johnson MedTech’s Pulsed Field ablation system. The fully integrated platform includes the VARIPULSE™ Catheter, TRUPULSE™ Generator, and CARTO™ 3 Mapping System VARIPULSE™ Software. The Platform is now approved for use in the United States, Europe, Japan and Canada, and has been used in External Evaluations to treat more than 1,000 patients worldwide.
Cardiovascular Solutions from Johnson & Johnson MedTech
Across Johnson & Johnson, we are tackling the world’s most complex and pervasive health challenges. Through a cardiovascular portfolio that provides healthcare professionals with advanced mapping and navigation, miniaturized tech, and precise ablation we are addressing conditions with significant unmet needs such as heart failure, coronary artery disease, stroke, and atrial fibrillation. We are the global leaders in heart recovery, circular restoration and the treatment of heart rhythm disorders, as well as an emerging leader in neurovascular care, committed to taking on two of the leading causes of death worldwide in heart failure and stroke. For more, visit biosensewebster.com and connect on LinkedIn and X, formerly Twitter.
About Johnson & Johnson
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity. Learn more about our MedTech sector’s global scale and deep expertise in cardiovascular, orthopaedics, surgery and vision solutions at https://thenext.jnjmedtech.com. Biosense Webster, Inc. is a Johnson & Johnson MedTech company.
Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding the VARIPULSE™ Platform. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Biosense Webster, Inc. and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: uncertainty of regulatory approvals; uncertainty of commercial success; challenges to patents; competition, including technological advances, new products and patents attained by competitors; product efficacy or safety concerns resulting in product recalls or regulatory action; changes to applicable laws and regulations, including global health care reforms; changes in behavior and spending patterns of purchasers of healthcare products and services; and trends toward healthcare cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at sec.gov, jnj.com or on request from Johnson & Johnson. Neither Biosense Webster, Inc. nor Johnson & Johnson does not undertake to update any forward-looking statement as a result of new information or future events or developments.
© Johnson & Johnson Biosense Webster, Inc. 2024. All rights reserved. US_BWI_THER_392304
_____________________________
i Peak primary effectiveness was defined in a post-hoc analysis as receiving 73-96 applications for PVI (n=85).
ii Precise in that it is marked on CARTO™ screen. When used with the CARTO™ 3 System
iii Complex ablation defined as AFIB procedures that include mapping and ablation.
iv Dr. DiBiase is an investigator for admIRE and was not compensated for his contributions to this announcement.
v Dr. Natale is a lead investigator for admIRE and was not compensated for her contributions to this announcement
1 Di Biase L, Zou F, Lin AN, et al. Feasibility of Three-Dimensional Artificial Intelligence Algorithm Integration with Intracardiac Echocardiography for Left Atrial Imaging During Atrial Fibrillation Catheter Ablation. Europace. 2023 Aug 2;25(9):euad211.
2 Reddy, V. Y. (2024). Pulsed field ablation to treat paroxysmal atrial fibrillation: Safety and effectiveness in the admire pivotal trial. Circulation, 150(15). https://doi.org/10.1161/circulationaha.124.070333
3 Debreceni D, Janosi K, Bocz B, et al. (2023). Zero fluoroscopy catheter ablation for atrial fibrillation: a systematic review and meta-analysis. Front
4 Rajendra A, Hunter TD, Morales GX, et al. (2023). Steerable sheath visualizable under 3D electroanatomical mapping facilitates paroxysmal atrial
5 Tahin T, Riba A, Nemeth B, et al. (2021). Implementation of a zero fluoroscopic workflow using a simplified intracardiac echocardiography guided method for catheter ablation of atrial fibrillation, including repeat procedures. BMC Cardiovasc Disord;21(1):407. doi: 10.1186/s12872-021-02219-8.
6 Mensah, G, Fuster, V, Murray, C. et al. Global Burden of Cardiovascular Diseases and Risks, 1990-2022. J Am Coll Cardiol. 2023 Dec, 82 (25) 2350–2473.
7 Staerk, et al. 2018 Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. doi: 10.1136_bmj.k1453 | BMJ 2018;361:k1453
8 Dilaveris PE, Kennedy HL. Silent atrial fibrillation: epidemiology, diagnosis, and clinical impact. Clin Cardiol. 2017;40:413–418.
9 Benjamin EJ, Go AS, Desvigne-Nickens P et al. Research Priorities in Atrial Fibrillation Screening: A Report From a National Heart, Lung, and Blood Institute Virtual Workshop. Circulation.
10 Natale, A. Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. Journal of the American College of Cardiology, 2014;64(7),647–656. doi: 10.1016/j.jacc.2014.04.072
Media contact:
Diane Pressman
[email protected]
Charlene DeBar
[email protected]
Investor Relations:
Tracy Menkowski
[email protected]
SOURCE Johnson & Johnson MedTech