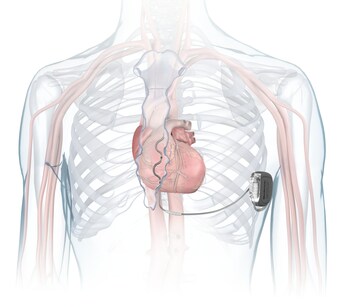
DUBLIN, Oct. 23, 2023 /PRNewswire/ — Medtronic plc (NYSE:MDT), a global leader in healthcare technology, has received U.S. Food and Drug Administration (FDA) approval for the Aurora EV-ICD™ MRI SureScan™ (Extravascular Implantable Cardioverter-Defibrillator) and Epsila EV™ MRI SureScan™ defibrillation lead to treat dangerously fast heart rhythms that can lead to sudden cardiac arrest (SCA). The Aurora EV-ICD system is the first-of-its-kind to provide the life-saving benefits of traditional, transvenous ICDs with a lead (thin wire) placed under the breastbone, outside of the heart and veins. The Aurora EV-ICD delivers lifesaving defibrillation, anti-tachycardia pacing (ATP), and back-up (pause-prevention) pacing therapies via a device similar in size, shape, and longevity to traditional, transvenous ICDs.
FDA approval of the Medtronic Aurora EV-ICD system includes the system’s proprietary procedure implant tools, and was supported by global pivotal trial results showing the system’s safety and effectiveness, which were published in The New England Journal of Medicine. In the coming weeks, the Aurora EV-ICD system will be commercially available on a limited basis in the United States.
“The Aurora EV-ICD system is a tremendous step forward in implantable defibrillator technology,” said Bradley P. Knight, M.D., medical director of Electrophysiology at Northwestern Medicine Bluhm Cardiovascular Institute and a co-author of the study. “Placing the leads outside of the heart, rather than inside the heart and veins, reduces the risk of long-term complications, ultimately allowing us to further evolve safe and effective ICD technology.”
In the pivotal study, the device’s effectiveness in delivering defibrillation therapy at implant was 98.7%, and there we no major intraprocedural complications, nor any unique complications observed related to the EV ICD procedure or system compared to transvenous and subcutaneous ICDs.[1] Additionally, 33 defibrillation shocks were avoided1 by having ATP – which paces the heart to interrupt and terminate a dangerous rhythm – programmed “on.” And at six months, 92.6% of patients (Kaplan-Meier estimate) were free from major system and/or procedure-related major complications such as hospitalization, system revision, or death.1
ICDs are highly effective in providing life-saving therapy for patients at risk of SCA, an electrical problem with the heart due to a dangerously fast heart rate (ventricular tachycardia) or irregular rhythm (ventricular fibrillation). If not treated immediately, SCA is often fatal (termed sudden cardiac death). Traditional ICDs typically are implanted below the collarbone, with the lead(s) threaded through the veins and into the heart.
“This FDA approval paves the way for patients to have a better overall experience with ICD therapy,” said Alan Cheng, M.D., chief medical officer of the Cardiac Rhythm Management business, which is part of the Cardiovascular Portfolio at Medtronic. “ICDs remain the gold standard for prevention of sudden cardiac death, and while the subcutaneous ICD avoids certain complications associated with transvenous defibrillators, it has limitations that may affect a patient’s comfort and quality-of-life. With the Aurora EV-ICD system, patients can benefit from the only ICD placed outside the vascular space that provides ATP and back-up pacing, in a device that is nearly half the size and with 60% greater projected battery longevity compared to the competitor’s subcutaneous ICD.”
The Aurora EV-ICD system is similar in size, shape, and longevity to traditional, transvenous ICDs. Unlike traditional ICDs, the Aurora EV-ICD system is implanted below the left armpit (in the left mid-axillary region) and the lead is placed under the breastbone (sternum) using a minimally invasive approach. The Epsila EV defibrillation lead is placed outside of the heart and veins, helping to avoid certain complications associated with transvenous leads, such as vascular injury and vessel occlusion (narrowing, blockage or compression of a vein).
The Aurora EV-ICD system includes features available in Medtronic transvenous ICDs, and offers additional advantages that are not available with the subcutaneous ICD including:
- Anti-tachycardia Pacing (ATP), to terminate ventricular arrhythmias (rapid and/or chaotic activity of the heart that can lead to SCA) using low-energy pacing pulses, potentially avoiding a defibrillation shock.
- Pause Prevention Pacing, to provide back-up pacing for brief, intermittent, heartbeat pauses.
- 40 Joule Defibrillation Energy, to deliver life-saving shocks in a device the size of transvenous ICDs (33 cc)
- Medtronic exclusive PhysioCurve™ design, to increase patient comfort and implant acceptance.
- 11.7-year projected longevity, to reduce device replacement procedures during a patient’s lifetime.
Patients who receive the commercially available Aurora device also will benefit from the addition of Smart Sense, a proprietary algorithm designed to reduce the potential for inappropriate shocks.
Medtronic will obtain real-world performance and safety data on the Aurora system in the Enlighten global post-approval registry, a prospective, non-randomized, observational, multicenter study, expected to last five years and enroll approximately 1,000 patients. First implants in Enlighten and first commercial implants worldwide were recently conducted by Lucas V.A. Boersma, M.D., Ph.D., cardiologist at St. Antonius Hospital, Nieuwegein, The Netherlands, and a limited launch is underway in select European countries.
The Aurora EV-ICD system is indicated for patients who are at risk of life-threatening arrhythmias, and who have not had a prior sternotomy and do not need chronic bradycardia (abnormally slow heartbeat) pacing.
About the EV ICD Pivotal Study
The EV ICD Pivotal study is a prospective, multicenter, single-arm, non-randomized, pre-market clinical study that assessed the safety and effectiveness of the Medtronic EV ICD system for patients at risk of sudden cardiac death. It enrolled 356 patients at 46 sites in 17 countries in North America, Europe, the Middle East, Asia, Australia and New Zealand, and results were published in The New England Journal of Medicine. Safety and effectiveness results were sustained out to 18 months.2
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across more than 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic’s periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
-end-
|
Contacts: |
|
|
Tracy McNulty |
Ryan Weispfenning |
|
Public Relations |
Investor Relations |
|
+1-763-526-2492 |
+1-763-505-4626 |