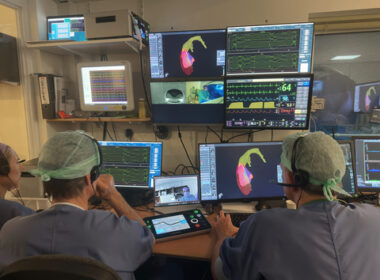
MINNEAPOLIS–(BUSINESS WIRE)–Imricor Medical Systems, Inc. (Company or Imricor) (ASX: IMR) is pleased to announce that it has commenced the VISABL-VT clinical trial by completing the first-in-human ventricular ablation guided by real-time MRI with the Company’s NorthStar Mapping System. The procedure was performed by the team at the Amsterdam University Medical Centre (AUMC), ranked […]
Other News
PrepMD OMNI: The Most Comprehensive CIED Software Solution, Now Listed in the Epic Showroom
BRAINTREE, Mass., April 23, 2025 /PRNewswire/ — PrepMD OMNI’s inclusion in the Epic Showroom establishes it as a trusted, integrated solution within one of the nation’s leading EHR ecosystems. This visibility gives hospitals and cardiac care centers across the country easier access to…
FLEX Vessel Prep™ System FLEX FIRST AV Registry 6-Month Data and the Hamburg Vessel Prep Experience Prior to Angioplasty presented at Charing Cross Symposium
MINNEAPOLIS, April 23, 2025 /PRNewswire/ — VentureMed Group, Inc., a privately held leader in medical device innovations for arteriovenous (AV) access and peripheral arterial disease (PAD), announced data presented at the Charing Cross Symposium, April 23 – 25th, London, England….
Orchestra BioMed Announces AVIM Therapy-Focused Satellite Symposium at HRS 2025 Annual Meeting
NEW HOPE, Pa., April 23, 2025 (GLOBE NEWSWIRE) — Orchestra BioMed Holdings, Inc. (Nasdaq: OBIO, “Orchestra BioMed” or the “Company”), a biomedical company accelerating high-impact technologies to patients through risk-reward sharing partnerships, today announced it will host an industry-sponsored satellite symposium at the Heart Rhythm Society (“HRS”) 2025 Annual Meeting, taking place April 24–27, 2025, in San Diego, California featuring recent advancements in the Company’s atrioventricular interval modulation (“AVIM”) therapy program. The April 25th, 6:45 am PT symposium titled “The Future of Cardiac Pacing: Unlocking the Potential of Atrioventricular Interval Modulation (AVIM) Therapy” will convene leading electrophysiologists, hypertension and heart failure specialists to discuss the unmet need in hypertension, AVIM therapy mechanism of action, and growing body of clinical evidence supporting this novel therapy for the treatment of patients with uncontrolled hypertension who have increased cardiovascular risk with or without an indication for a pacemaker.
THE HEART RHYTHM SOCIETY ANNOUNCES FRAMEWORK FOR ESTABLISHING ATRIAL FIBRILLATION CENTERS OF EXCELLENCE AND KEY OPERATIONAL STANDARDS
WASHINGTON, April 23, 2025 /PRNewswire/ — Today, the Heart Rhythm Society (HRS) released a framework outlining criteria for establishing an Atrial Fibrillation (AF) Center of Excellence (CoE) and key operational standards to provide multidisciplinary care for AF patients. AF, the most…
Conavi Medical Corp. Announces Closing of $20M Public Offering
NOT FOR DISTRIBUTION TO U.S. NEWS WIRE SERVICES OR FOR DISSEMINATION IN THE UNITED STATES TORONTO, April 23, 2025 (GLOBE NEWSWIRE) — Conavi Medical Corp. (TSXV: CNVI; OTC: CNVIF) (“Conavi” or the “Company”), a commercial stage medical device company focused on designing, manufacturing, and marketing imaging technologies to guide common minimally invasive cardiovascular procedures, is pleased to announce that it has closed its previously announced, upsized equity offering for aggregate gross proceeds of $20 million (the “Offering”). The net proceeds from the Offering will be used to advance and complete the development and pre-clinical testing of its Novasight 3.0 technology, with the goal of submitting a 510(k) clearance application to the U.S. Food and Drug Administration in Q3 of 2025. The Company also intends to use the net proceeds for working capital and other general corporate purposes. Bloom Burton Securities Inc. acted as sole and exclusive agent for the Offering. Under the Offering, subscribers either purchased common shares at $0.40 per common share (the “Common Shares”) or pre-funded common share purchase warrants for $0.39999 per pre-funded common share purchase warrant (“Pre-Funded Warrants” and, together with the Common Shares, the “Securities”). Investors purchased a total of 50,000,000 Securities (consisting of 32,500,000 Common Shares and 17,500,000 Pre-Funded Warrants) for gross proceeds of $20 million. Each Pre-Funded Warrant issued in lieu of a Common Share at the election of a subscriber entitles the holder thereof to acquire one Common Share at an exercise price of $0.00001 per Common Share. The Pre-Funded Warrants will not expire. In Canada, the Securities purchased pursuant to the Offering were qualified for sale by way of a short form prospectus dated April 15, 2025, which was filed in British Columbia, Alberta and Ontario. The Securities were purchased by way of private placement in the United States, pursuant to exemptions from the registration requirements under the U.S. Securities Act of 1933 (the “U.S. Securities Act”), and pursuant to all applicable U.S. state securities laws. In addition, the Securities were also sold by way of private placement in certain other jurisdictions outside of Canada and the United States pursuant to and in compliance with applicable securities laws. CPOINT Capital Corp., an insider of the Company, purchased 625,000 Common Shares under the Offering and Juno Pharmaceuticals LP, an insider of the Company, purchased 1,250,000 Common Shares under the Offering. The subscriptions for Common Shares by CPOINT Capital Corp. and Juno Pharmaceuticals LP are related party transactions within the meaning of applicable Canadian securities laws. The subscriptions by such insiders are exempt from the formal valuation and minority approval requirements applicable to related party transactions on the basis that the value of the transactions insofar as they involve related parties is less than 25% of the Company’s market capitalization. The Board of Directors of the Company has approved the Offering. A material change report in respect of the related party transactions could not be filed earlier than 21 days prior to the closing of the Offering due to the limited time between the commitment by such insiders to purchase the subject Common Shares and the closing of the Offering. The securities described herein have not been, and will not be, registered under the U.S. Securities Act, or any U.S. state securities laws, and accordingly, may not be offered or sold to, or for the account or benefit of, persons in the United States or to U.S. Persons (as such terms are defined in Regulation S under the U.S. Securities Act), except in compliance with the registration requirements of the U.S. Securities Act and applicable U.S. state securities requirements or pursuant to exemptions therefrom. This press release does not constitute an offer to sell or a solicitation of an offer to buy any of the Company’s securities. About Conavi Medical Conavi Medical is focused on designing, manufacturing, and marketing imaging technologies to guide common minimally invasive cardiovascular procedures. Its patented Novasight Hybrid™ System is the first system to combine both intravascular ultrasound (IVUS) and optical coherence tomography (OCT) to enable simultaneous and co-registered imaging of coronary arteries. The Novasight Hybrid System has 510(k) clearance from the U.S. Food and Drug Administration; and regulatory approval for clinical use from Health Canada, China’s National Medical Products Administration, and Japan’s Ministry of Health, Labor and Welfare. For more information, visit http://www.conavi.com/. Notice on forward-looking statements: This press release includes forward-looking information or forward-looking statements within the meaning of applicable securities laws regarding Conavi and its business, which may include, but are not limited to, statements with respect to the anticipated use of proceeds from the Offering. All statements that are, or information which is, not historical facts, including without limitation, statements regarding future estimates, plans, programs, forecasts, projections, objectives, assumptions, expectations or beliefs of future performance, are “forward-looking information or statements”. Often but not always, forward-looking information or statements can be identified by the use of words such as “shall”, “intends”, “anticipate”, “believe”, “plan”, “expect”, “intend”, “estimate” “anticipate” or any variations (including negative variations) of such words and phrases, or state that certain actions, events or results “may”, “might”, “can”, “could”, “would” or “will” be taken, occur, lead to, result in, or, be achieved. Such statements are based on the current expectations and views of future events of the management of the Company. They are based on assumptions and subject to risks and uncertainties. Although management believes that the assumptions underlying these statements are reasonable, they may prove to be incorrect. The forward-looking events and circumstances discussed in this release, may not occur and could differ materially as a result of known and unknown risk factors and uncertainties affecting the Company, including, without limitation, those listed in the “Risk Factors” section of the short form prospectus dated April 15, 2025 and the joint information circular of the Company dated August 30, 2024 (both of which are on the Company’s profile at www.sedarplus.ca). Although Conavi has attempted to identify important factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other factors that cause actions, events or results to differ from those anticipated, estimated or intended. Accordingly, readers should not place undue reliance on any forward-looking statements or information. No forward-looking statement can be guaranteed. Except as required by applicable securities laws, forward-looking statements speak only as of the date on which they are made and Conavi does not undertake any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events, or otherwise. No regulatory authority has approved or disapproved the content of this press release. Neither the TSX Venture Exchange nor its Regulatory Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this press release. CONTACT: CONTACT:
Stefano Picone
Chief Financial Officer
(416) 483-0100
FIELD MEDICAL ANNOUNCES FIRST-IN-HUMAN DATA FOR FOCAL PFA IN SCAR-RELATED VT AT HEART RHYTHM 2025
Eight presentations, including late-breaking science, live cases, and a landmark symposium CARDIFF-BY-THE-SEA, Calif., April 23, 2025 /PRNewswire/ — Field Medical Inc., a leader in cardiac pulsed field ablation (PFA) technology, announced today its FieldForce™ Ablation System will be…
Catheter Precision to Acquire Assets in Development for Late-Stage Treatment for Acute Decompensated Heart Failure
Initial Clinical Studies Completed with Significant Positive Results
Kestra Medical Technologies to Showcase Innovation in Sudden Cardiac Arrest Protection and Recovery at Heart Rhythm 2025
Company to spotlight ASSURE WCD performance and expanded clinical applicationsKIRKLAND, Wash., April 23, 2025 (GLOBE NEWSWIRE) — Kestra Medical Technologies, Ltd. (Nasdaq: KMTS) (“Kestra”), a wearable medical device and digital healthcare company, announced today it will exhibit at Heart Rhythm 2025, the annual meeting of the Heart Rhythm Society (HRS), taking place April 24-27 at the San Diego Convention Center. This marks Kestra’s first major industry showcase following its successful IPO earlier this year. Kestra will debut an immersive in-booth experience designed to bring the ASSURE® system to life—demonstrating how this innovative technology is redefining protection for patients at risk of sudden cardiac arrest. By combining lifesaving defibrillation therapy with intuitive, intelligent, and connected diagnostic and patient support capabilities, the ASSURE system is a key part of a broader vision for a holistic cardiac care ecosystem that supports patients and providers across the recovery journey. “Our presence at Heart Rhythm 2025 comes at a pivotal time for Kestra,” said Brian Webster, President and Chief Executive Officer of Kestra. “Following our IPO, we’re moving forward with increasing momentum—and this year’s HRS meeting is an opportunity to demonstrate how the ASSURE system goes beyond protection to offer a smarter, more connected recovery experience for both patients and care teams.” In addition to exhibiting, Kestra will also present new real-world clinical data highlighting the impact of the ASSURE system. Kestra is also proud to sponsor the Women in EP Luncheon for the fourth consecutive year—underscoring its ongoing commitment to leadership, innovation, and equity in cardiovascular care. About KestraKestra Medical Technologies, Ltd. is a commercial-stage wearable medical device and digital healthcare company focused on transforming patient outcomes in cardiovascular disease using monitoring and therapeutic intervention technologies that are intuitive, intelligent, and connected. For more information, please visit www.kestramedical.com. Media contactRhiannon Pickusrhiannon.pickus@kestramedical.com Investor contactNeil Bhalodkarneil.bhalodkar@kestramedical.com
Roche receives CE Mark for its Chest Pain Triage algorithm to enhance detection of Acute Coronary Syndrome (ACS)
Roche, in collaboration with Universitätsklinikum Heidelberg, has developed a Chest Pain Triage algorithm – a CE-marked IVD medical device1 set to transform cardiac careThis novel algorithm offers a standardised assessment, helping emergency room doctors to make confident clinical decisions in ruling in or ruling out heart attacks (acute myocardial infarction)Cardiovascular disease causes a third of worldwide deaths2, with chest pain being the second highest reason for emergency department (ED) visits3 Basel, 23 April 2025 – Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today the introduction of its innovative Chest Pain Triage algorithm as part of the navifyⓇ Algorithm Suite. This groundbreaking algorithm is designed to more quickly and accurately detect Acute Coronary Syndrome (ACS) in patients presenting with chest pain, one of the most common reasons for Emergency Department (ED) visits. As EDs are typically one of the most crowded hospital units, leading to challenges to swiftly diagnose critical conditions, such as chest pain.4 This new algorithm was developed in collaboration with Universitätsklinikum Heidelberg. The Chest Pain Triage algorithm leverages state-of-the-art diagnostic technologies, including high-sensitivity cardiac troponin testing, to provide healthcare professionals with timely and reliable data to differentiate between cardiac and non-cardiac chest pain. This advanced algorithm is part of a wider integrated offering from Roche to address ACS, including the use of the cardiac troponin T Assay and integration with existing lab solutions, offering an efficient and comprehensive approach to patient triage in emergency settings. The Chest Pain Triage algorithm also leverages the European Society of Cardiology’s (ESC) guidelines, and leading cardiologists and emergency medicine experts contributed to the development of the algorithm. “The introduction of our Chest Pain Triage algorithm underscores Roche’s commitment to improving care for cardiovascular disease, one of the world’s largest health burdens,” said Matt Sause, CEO, Roche Diagnostics. “One of the major challenges in managing chest pain in the emergency department is the length of stay, especially since some patients aren’t actually having a heart attack. Our Chest Pain Triage algorithm can help doctors quickly decide who needs urgent cardiac care and who could be discharged sooner. With an early rule-out pathway, we can cut down Emergency Department visit times by over three hours.5” The new algorithm aims to identify patients genuinely at risk by accurately identifying non-cardiac chest pain cases through a definitive Rule-In, Rule-Out or Observe recommendation according to the ESC guidelines. The algorithm simplifies decision making by automatically selecting the proper ESC 0/1, 0/2, or 0/3 accelerated pathway, based on the timing of the blood sample collection. This has the potential to reduce unnecessary hospital admissions and associated costs. The algorithm also expedites the treatment process for patients with true ACS with Rapid Assessments of chest pain onset more than three hours before the first blood sample. In addition, it includes a medical dossier for clinician support, and simplifies documentation by allowing doctors to easily copy recommendations and results into patient records. For more information, please visit the Chest Pain Triage algorithm site. The development of the Chest Pain Triage algorithm is part of Roche’s commitment to early identification and treatment of Cardiovascular disease. The algorithm is available in Europe, the Middle East, and Asia, with availability in the United States at a later date, through Roche’s navify Algorithm Suite, and can be integrated into current emergency department workflows*. Roche’s cardiometabolic portfolio supports faster and more accurate triage decisions, and future ACS offerings will combine next-generation digital algorithms, biomarkers, near patient care devices, and laboratory analyzers. The navify Algorithm Suite is a cloud-based platform hosting clinical algorithms from Roche and partners. It provides labs and hospitals with direct workflow integration through Electronic Health Records (EHR) and Lab Information Systems (LIS) for faster and more efficient processes. About navify The navify portfolio from Roche includes more than 30 digital solutions for labs, hospitals and patients worldwide. navify solutions connect the healthcare community with a robust digital infrastructure to integrate data efficiently and to accelerate clinician access to innovations as well as operational and medical insights. This work includes collaborating with other innovative companies such as Fortinet in cybersecurity services. The navify platform is designed to deliver security at every step of the data analytical process. All data is encrypted at rest and in transit. The solution is operated in compliance with applicable laws and regulations in the USA with HIPAA (Health Insurance Portability and Accountability) as well as with GDPR (General Data Protection Regulation) regulations in Europe. Healthcare professionals can visit navify Marketplace to browse and request a growing number of next generation digital solutions from Roche and other companies — all designed to drive operational and clinical excellence, built on the foundational pillars of digital trust. More information is also available at navify.com. About Roche Founded in 1896 in Basel, Switzerland, as one of the first industrial manufacturers of branded medicines, Roche has grown into the world’s largest biotechnology company and the global leader in in-vitro diagnostics. The company pursues scientific excellence to discover and develop medicines and diagnostics for improving and saving the lives of people around the world. We are a pioneer in personalised healthcare and want to further transform how healthcare is delivered to have an even greater impact. To provide the best care for each person we partner with many stakeholders and combine our strengths in Diagnostics and Pharma with data insights from the clinical practice. For over 125 years, sustainability has been an integral part of Roche’s business. As a science-driven company, our greatest contribution to society is developing innovative medicines and diagnostics that help people live healthier lives. Roche is committed to the Science Based Targets initiative and the Sustainable Markets Initiative to achieve net zero by 2045. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan. For more information, please visit www.roche.com. All trademarks used or mentioned in this release are protected by law. * Availability will depend on the specific market and country in some cases. Please consult a local Roche representative for the availability of the chest pain algorithm in your market References [1] European Union: EUR-Lex. Chest Pain Triage algorithm registration [Internet: accesses March 24, 2025] Chest Pain Triage algorithm is an in-vitro diagnostic (IVD) medical device CE-marked (NB 0123) under the requirements laid out in the IVD regulation (EU) 2017/746 (IVDR). [2] Saloni D et al. Cardiovascular Diseases. [Internet: accessed March 24, 2025]. Available from: https://ourworldindata.org/cardiovascular-diseases?insight=cardiovascular-diseases-are-the-most-common-cause-of-death-worldwide#all-charts [3] Yukselen Z, Majmundar V, Dasari M, Arun Kumar P, Singh Y. Chest Pain Risk Stratification in the Emergency Department: Current Perspectives. Open Access Emerg Med. 2024 Feb 4;16:29-43. doi: 10.2147/OAEM.S419657. PMID: 38343728; PMCID: PMC10853047 [4] Sartini M, et al. Overcrowding in Emergency Department: Causes, consequences, and solutions – a narrative review. Healthcare (Basel). 2022 Aug;10(9):1625) [5] Anand A, et al. High-sensitivity cardiac troponin on presentation to rule out myocardial infarction: A stepped-wedge cluster randomized controlled trial. Circulation. 2021 Jun;143(23):2214-2224) Roche Global Media Relations Phone: +41 61 688 8888 / e-mail: media.relations@roche.com Hans Trees, PhD Phone: +41 79 407 72 58 Sileia Urech Phone: +41 79 935 81 48 Nathalie Altermatt Phone: +41 79 771 05 25 Lorena Corfas Phone: +41 79 568 24 95 Simon Goldsborough Phone: +44 797 32 72 915 Karsten Kleine Phone: +41 79 461 86 83 Nina Mählitz Phone: +41 79 327 54 74 Kirti Pandey Phone: +49 172 6367262 Yvette Petillon Phone: +41 79 961 92 50 Dr Rebekka Schnell Phone: +41 79 205 27 03
Attachment
23042025_MR_Roche navify chest pain_en