FLAGSTAFF, Ariz., June 4, 2019 /PRNewswire/ — W. L. Gore & Associates (Gore) announced the U.S. Food and Drug Administration’s (FDA’s) premarket approval (PMA) of the GORE® CARDIOFORM ASD Occluder for the percutaneous closure of ostium secundum atrial septal defects (ASDs). The FDA approval was supported by data collected from the pivotal stage of the Gore […]
Other News
AngioDynamics Completes Sale of NAMIC® Fluid Management Portfolio to Medline Industries, Inc.
LATHAM, N.Y.–(BUSINESS WIRE)–AngioDynamics, Inc. (NASDAQ: ANGO), a leading provider of innovative, minimally invasive medical devices for vascular access, peripheral vascular disease, and oncology, today announced that it closed the previously announced sale of its NAMIC® fluid management portfolio to Medline Industries, Inc. on May 31, 2019 for $167.5 million. AngioDynamics has […]
BioSig Technologies Receives $4.6 Million in Cash Warrant and Option Exercises
Email Print Friendly Share June 03, 2019 07:52 ET | Source: BioSig Technologies, Inc. Santa Monica, CA, June 03, 2019 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (NASDAQ: BSGM), a medical technology company developing a proprietary biomedical signal processing platform designed to address an unmet technology need for the electrophysiology (EP) marketplace, today announced that it received a […]
Ancora Heart Enrolls First Patient in U.S. Early Feasibility Study of First-of-Its-Kind Investigational Heart Failure Therapy
SANTA CLARA, Calif.–(BUSINESS WIRE)–Ancora Heart, Inc., a company developing a novel therapy to address heart failure, today announced the first patient was enrolled in a U.S. early feasibility study evaluating the AccuCinch® Ventricular Repair System as a treatment for patients with reduced ejection fraction systolic heart failure (HFrEF). The first patient […]
CHF Solutions, Inc. Reports Inducement Grants Under NASDAQ Listing Rule 5635(c)(4)
EDEN PRAIRIE, Minn., May 29, 2019 (GLOBE NEWSWIRE) — CHF Solutions, Inc. (NASDAQ: CHFS), today announced that, on May 23, 2019, the independent directors approved five equity awards under CHF Solution’s New-Hire Equity Incentive Plan, as material inducements to five individuals entering into employment with the Company. The equity awards […]
Safety and efficacy of Philips’ Stellarex .035 low-dose drug-coated balloon demonstrated in clinical trials at three years
Stellarex is the only low-dose [1] drug-coated balloon (DCB) to demonstrate a significant treatment effect and high safety profile through 3 years ILLUMENATE Pivotal trial showcases durable primary patency (maintained blood flow) in the most complex patient pool studied in a DCB randomized clinical trial No significant difference in mortality […]
Medtronic Gains U.S. FDA Clearance for New Catheter System for HIS Bundle Pacing
DUBLIN – May 29, 2019 – Medtronic plc (NYSE:MDT) today announced U.S. Food and Drug Administration (FDA) clearance and commercial launch for the SelectSite(TM) C304-HIS deflectable catheter system for use in procedures involving His-bundle pacing (HBP). The SelectSite C304-HIS deflectable catheter system features a deflectable, out-of-plane curve to reach the bundle […]
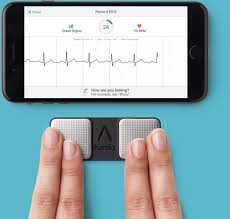
AliveCor and Best Buy Partner to Bring KardiaMobile to Select Stores Nationwide
MOUNTAIN VIEW, Calif., May 29, 2019 /PRNewswire/ — AliveCor, the leader in FDA-cleared consumer electrocardiogram (ECG) technology, and Best Buy Co., Inc. (NYSE: BBY) today announced a strategic partnership, bringing AliveCor’s products to select Best Buy stores across the country. This is the first time AliveCor’s products will be sold in consumer electronics stores […]
Intact Vascular Announces $25 Million Financing with Vensana Capital to Drive Commercialization of the Tack Endovascular System®
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced a $25 million financing with Vensana Capital as the lead investor. Vensana was joined by existing investors including New Enterprise Associates (“NEA”), H.I.G. BioHealth Partners, and Quaker Partners. The Company reported closure […]
Vesper Medical Announces $37 Million Financing with Vensana Capital and Gilde Healthcare to Complete Development of Its Duo Venous Stent System™
WAYNE, Pa.–(BUSINESS WIRE)–Vesper Medical, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced a $37 million financing with Vensana Capital and Gilde Healthcare as lead investors. They were joined by existing investors New Enterprise Associates (“NEA”) and Quaker Partners. The first tranche of the financing […]