SANTA CLARA, Calif.–(BUSINESS WIRE)–Ancora Heart, Inc., a company developing a novel therapy to address heart failure, today announced the first patient was enrolled in a U.S. early feasibility study evaluating the AccuCinch® Ventricular Repair System as a treatment for patients with reduced ejection fraction systolic heart failure (HFrEF). The first patient […]
Other News
CHF Solutions, Inc. Reports Inducement Grants Under NASDAQ Listing Rule 5635(c)(4)
EDEN PRAIRIE, Minn., May 29, 2019 (GLOBE NEWSWIRE) — CHF Solutions, Inc. (NASDAQ: CHFS), today announced that, on May 23, 2019, the independent directors approved five equity awards under CHF Solution’s New-Hire Equity Incentive Plan, as material inducements to five individuals entering into employment with the Company. The equity awards […]
Safety and efficacy of Philips’ Stellarex .035 low-dose drug-coated balloon demonstrated in clinical trials at three years
Stellarex is the only low-dose [1] drug-coated balloon (DCB) to demonstrate a significant treatment effect and high safety profile through 3 years ILLUMENATE Pivotal trial showcases durable primary patency (maintained blood flow) in the most complex patient pool studied in a DCB randomized clinical trial No significant difference in mortality […]
Medtronic Gains U.S. FDA Clearance for New Catheter System for HIS Bundle Pacing
DUBLIN – May 29, 2019 – Medtronic plc (NYSE:MDT) today announced U.S. Food and Drug Administration (FDA) clearance and commercial launch for the SelectSite(TM) C304-HIS deflectable catheter system for use in procedures involving His-bundle pacing (HBP). The SelectSite C304-HIS deflectable catheter system features a deflectable, out-of-plane curve to reach the bundle […]
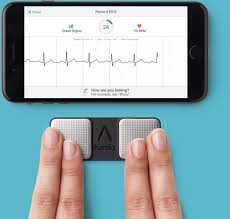
AliveCor and Best Buy Partner to Bring KardiaMobile to Select Stores Nationwide
MOUNTAIN VIEW, Calif., May 29, 2019 /PRNewswire/ — AliveCor, the leader in FDA-cleared consumer electrocardiogram (ECG) technology, and Best Buy Co., Inc. (NYSE: BBY) today announced a strategic partnership, bringing AliveCor’s products to select Best Buy stores across the country. This is the first time AliveCor’s products will be sold in consumer electronics stores […]
Intact Vascular Announces $25 Million Financing with Vensana Capital to Drive Commercialization of the Tack Endovascular System®
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced a $25 million financing with Vensana Capital as the lead investor. Vensana was joined by existing investors including New Enterprise Associates (“NEA”), H.I.G. BioHealth Partners, and Quaker Partners. The Company reported closure […]
Vesper Medical Announces $37 Million Financing with Vensana Capital and Gilde Healthcare to Complete Development of Its Duo Venous Stent System™
WAYNE, Pa.–(BUSINESS WIRE)–Vesper Medical, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced a $37 million financing with Vensana Capital and Gilde Healthcare as lead investors. They were joined by existing investors New Enterprise Associates (“NEA”) and Quaker Partners. The first tranche of the financing […]
HeartFlow Names Dana G. Mead, Jr. as President and CEO
REDWOOD CITY, Calif.–(BUSINESS WIRE)–HeartFlow, Inc. today announced that it has named Dana G. Mead, Jr. as President and Chief Executive Officer (CEO). Mead also has been appointed to HeartFlow’s Board of Directors. Former President and CEO John H. Stevens, M.D., will continue to serve on the Board of Directors and […]
Novoheart to Acquire Xellera Therapeutics
Xellera Therapeutics Limited will accelerate the progression of Novoheart’s current business scope Significantly enhances Novoheart’s liquidity, gaining access to approximately C$22,500,000 cash VANCOUVER, British Columbia, May 28, 2019 (GLOBE NEWSWIRE) — Novoheart Holdings Inc. (“Novoheart” or the “Company”) (TSXV: NVH; FWB: 3NH) announced that effective on May 27, 2019, it has entered into […]
MyoKardia Presents Results from Phase 1a Clinical Trial of MYK-491 at the European Society of Cardiology Heart Failure Congress in Athens, Greece
First-in-Human Data for MYK-491 in Healthy Volunteers Demonstrated Well-Tolerated Increases in Cardiac Contractility Topline Results from Phase 2a Clinical Trial of MYK-491 in Patients with Stable Heart Failure with Reduced Ejection Fraction Anticipated in Second Half 2019 SOUTH SAN FRANCISCO, Calif. and ATHENS, Greece, May 28, 2019 (GLOBE NEWSWIRE) — […]