Santa Monica, CA, Oct. 29, 2018 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (NASDAQ: BSGM) (“BioSig” or the “Company”), a medical device company developing a proprietary biomedical signal processing platform designed to address an unmet technology need for the $4.6 billion electrophysiology (EP) marketplace, announced today that it will be presenting […]
Other News
InventHelp Inventor Develops Dual-Purpose Angiographic Medical Instrument (SOG-259)
PITTSBURGH, Oct. 30, 2018 /PRNewswire/ — An inventor from Roseville, Calif., has developed the MULTI-PURPOSE ANGIOGRAPHIC CATHETER SYSTEM, a medical instrument that will provide enhanced angiographic capabilities. “As a physician, I wanted to develop a way to both inject contrast and advance a wire into areas I desired to reach,” said the inventor. The […]
Teleflex Receives 2018 Medical Design Excellence Award (MDEA) for Arrow® AC3 OptimusTM Intra-Aortic Balloon Pump
WAYNE, Pa.–(BUSINESS WIRE)–Teleflex Incorporated (NYSE: TFX), a leading global provider of medical technologies for critical care and surgery, is proud to announce the Arrow AC3 Optimus Intra-Aortic Balloon Pump, has been selected as the bronze winner in the cardiovascular device category of the 20th annual Medical Design Excellence Awards competition. The […]
Acer Therapeutics Submits NDA for EDSIVO™ for the Treatment of vEDS
NEWTON, Mass., Oct. 29, 2018 (GLOBE NEWSWIRE) — Acer Therapeutics Inc. (Nasdaq: ACER), a pharmaceutical company focused on the acquisition, development and commercialization of therapies for serious rare and ultra-rare diseases with critical unmet medical need, today announced that it has submitted a New Drug Application (NDA) to the U.S. […]
Esperion Announces Positive Top-Line Results from Final Pivotal Phase 3 Study of Bempedoic Acid
ANN ARBOR, Mich., Oct. 28, 2018 (GLOBE NEWSWIRE) — Esperion (NASDAQ: ESPR) today announced positive top-line results from its global, pivotal Phase 3 clinical study (Study 2 or 1002-047). This trial was a 52-week, randomized, double-blind, placebo-controlled study to evaluate the LDL-C lowering efficacy and the safety and tolerability of […]
Fujifilm Earns Highest MD Buyline Ratings Across Multiple Categories And Devices In Digital Radiography And Endoscopy
STAMFORD, Conn., Oct. 29, 2018 /PRNewswire/ — FUJIFILM Medical Systems U.S.A., Inc., a leading provider of diagnostic imaging and endoscopic imaging solutions, has earned top ratings in MD Buyline’s User Satisfaction Ratings for several of its digital radiography (DR) solutions and endoscopic systems in the third quarter of 2018. These latest rankings—based directly on input from […]
Correvio Announces Brinavess Eligibility for Patent Extension
VANCOUVER, Oct. 29, 2018 /PRNewswire/ – Correvio Pharma Corp. (NASDAQ: CORV /TSX: CORV), a specialty pharmaceutical company focused on providing high-quality brands to acute care physicians and patients, today announced that it has received independent regulatory and legal opinions that Brinavess® (vernakalant hydrochloride, IV) may qualify for up to a 5-year patent extension from the U.S. […]
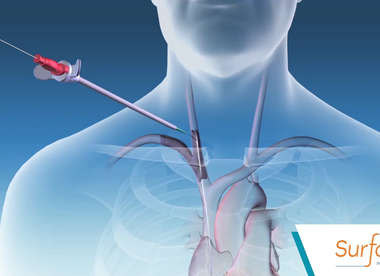
Bluegrass Vascular Announces Positive Results From Independent Study Building On Growing Body Of Evidence For Clinical Application Of The Surfacer System
SAN ANTONIO, Oct. 27, 2018 /PRNewswire/ — Bluegrass Vascular Technologies (Bluegrass Vascular), a private medical technology company focused on innovating lifesaving devices and methods for vascular access procedures, announced today its Surfacer® Inside-Out® Access Catheter System has demonstrated positive commercial use, consistently achieving central venous access in patients with upper body occlusions. The study, […]
Inositec’s INS-3001 Significantly Reduces Vascular Calcification Data Presented at Kidney Week 2018 Indicates
ZURICH–(BUSINESS WIRE)–Inositec, a pioneer in the development of life-saving small molecule drugs based on myo-inositol hexaphosphate (IP6), announced today that INS-3001, a novel vascular calcification inhibitor, demonstrated the ability to significantly inhibit the calcification process in preclinical studies. The build-up of calcium deposits in arterial walls and cardiac valves lead to […]
Amarin Schedules Webcast Discussion of Primary REDUCE-IT™ Trial Results Following Presentation at 2018 Scientific Sessions of American Heart Association
BEDMINSTER, N.J. and DUBLIN, Ireland, Oct. 26, 2018 (GLOBE NEWSWIRE) — Amarin Corporation plc (NASDAQ:AMRN), a pharmaceutical company focused on the commercialization and development of therapeutics to improve cardiovascular health, looks forward to the presentation of primary results of Amarin’s landmark cardiovascular outcomes study of Vascepa®(icosapent ethyl), the REDUCE-IT™ study, as […]