DUBLIN – May 2, 2019 – Medtronic plc (NYSE:MDT) today announced that the company has received U.S. Food and Drug Administration (FDA) approval for the CareLink SmartSync(TM) Device Manager. With the introduction of SmartSync, physicians will now be able to use an Apple® iPad® to program and manage data from Medtronic’s BlueSync-enabled […]
Other News
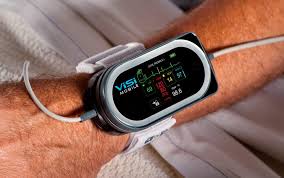
Sotera Wireless to Showcase Its Life-Saving ViSi Mobile 1.5G at the 2019 IHI Patient Safety Congress
SAN DIEGO–(BUSINESS WIRE)–Sotera Wireless, maker of the ViSi Mobile® Surveillance Monitoring System will be exhibiting at the Institute for Healthcare Improvement Patient Safety Congress (IHI) on May 15-17 in Houston, TX. The IHI Patient Safety Congress brings together people who are passionate about providing the highest quality care and patient […]
GENETIC-AF Phase 2B Trial Results Published in the Journal of American College of Cardiology: Heart Failure
WESTMINSTER, Colo., May 01, 2019 (GLOBE NEWSWIRE) — ARCA biopharma, Inc. (Nasdaq: ABIO), a biopharmaceutical company applying a precision medicine approach to developing genetically-targeted therapies for cardiovascular diseases, today announced that the paper “GENETIC-AF: Bucindolol for the Maintenance of Sinus Rhythm in a Genotype-Defined Heart Failure Population” was published in JACC: Heart Failure, a journal […]
Biofourmis’ RhythmAnalytics™ Platform Receives FDA Clearance for AI-Based Automated Interpretation of Cardiac Arrhythmias
BOSTON, April 30, 2019 /PRNewswire/ — Biofourmis announced today that the U.S. Food and Drug Administration (FDA) has granted the company clearance for its RhythmAnalyticsTM platform, a cloud-based software for automated interpretation of cardiac arrhythmias. It uses an enhanced deep-learning technique to detect over 15 types of cardiac arrhythmias along with beat-by-beat morphology computation which […]
Zynex Announces 2019 First Quarter Earnings
ENGLEWOOD, Colo., April 30, 2019 /PRNewswire/ — Zynex, Inc. (NASDAQ: ZYXI), an innovative medical technology company specializing in the manufacture and sale of non-invasive medical devices for pain management, stroke rehabilitation, cardiac monitoring and neurological diagnostics, today reported financial results for its first quarter ended March 31, 2019. First Quarter Financial Results Summary:For the first […]
Omron Healthcare Launches Complete™, the First Blood Pressure Monitor with EKG Capability in a Single Device, for Retail Sale
LAKE FOREST, Ill., May 1, 2019 /PRNewswire/ — Omron Healthcare, Inc., the number one doctor and pharmacist recommended blood pressure brand, today launched Complete™, the first blood pressure monitor with EKG capability in a single device, for retail sale in the U.S. online at OmronHealthcare.com. Complete recently received FDA clearance as a medical device. […]
CryoLife Reports First Quarter 2019 Financial Results
ATLANTA, April 30, 2019 /PRNewswire/ — First Quarter and Recent Business Highlights: Total revenues were $67.5 million in the first quarter of 2019, reflecting year over year growth of 9% and an 11% increase on a non-GAAP constant currency basis, both compared to the first quarter of 2018 On-X® revenues increased 14%, and 15% on […]
LivaNova Reports First Quarter 2019 Results
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN), a market-leading medical technology and innovation company, today reported results for the quarter ended March 31, 2019. For the first quarter of 2019, worldwide sales from continuing operations were $250.8 million, an increase of 0.2 percent on a reported basis and an increase of 4.2 percent on […]
Medtronic Receives FDA Approval for World’s First Quadripolar Active Fixation Left Heart Lead
DUBLIN – May 1, 2019 – Medtronic plc (NYSE:MDT) announced it has received U.S. Food and Drug Administration (FDA) approval for the Attain Stability(TM) Quad MRI SureScan(TM) left heart lead. Paired with Medtronic quadripolar cardiac resynchronization therapy-defibrillators (CRT-D) and -pacemakers (CRT-P), the Attain Stability Quad lead is the only active-fixation left […]
NuCryo Vascular Announces Series B Financing and the Launch of its Extended PolarCath Catheter
SAN JOSE, Calif.–(BUSINESS WIRE)–NuCryo Vascular, the manufacturer and marketer of the PolarCath Balloon Dilatation System, announced today that the company is raising a Series B round of financing and is launching its Extended PolarCath Balloon Dilatation Catheter. The Series B financing will provide the necessary working capital to support the […]