NEW YORK and MELBOURNE, Australia, Jan. 14, 2019 (GLOBE NEWSWIRE) — Mesoblast Limited (ASX: MSB; Nasdaq: MESO) today announced that it has drawn down a further US$15 million from its US$75 million, non-dilutive, four-year credit facility with Hercules Capital, Inc. (NYSE:HTGC). The funds will be used primarily to ramp up […]
Other News
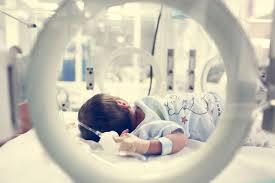
FDA Approves World’s First Device for Treatment of Premature Babies and Newborns with an Opening in Their Hearts (a Common Congenital Defect)
ABBOTT PARK, Ill., Jan. 14, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced the U.S. Food and Drug Administration (FDA) approved the Amplatzer Piccolo™ Occluder, the world’s first medical device that can be implanted in the tiniest babies (weighing as little as two pounds) using a minimally invasive procedure to treat patent ductus arteriosus, or […]
Shockwave Initiates U.S. Pivotal Study for Coronary Intravascular Lithotripsy
SANTA CLARA, Calif., Jan. 14, 2019 (GLOBE NEWSWIRE) — Shockwave Medical, Inc., a pioneer in the development and commercialization of Intravascular Lithotripsy (IVL) to treat complex calcified cardiovascular disease, has initiated its U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) study – DISRUPT CAD III – for the […]
Endologix Appoints John D. Zehren as Chief Commercial Officer
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (Nasdaq:ELGX) (“Endologix” or the “Company”), a developer and marketer of innovative treatments for aortic disorders, today announced that it has appointed John D. Zehren as the Company’s Chief Commercial Officer, effective as of January 7, 2019 (the “Effective Date”). Mr. Zehren brings to Endologix more than […]
Matinas BioPharma Announces a Research Evaluation with Top Global Pharma Company Based on Its Proprietary Drug Delivery Platform
BEDMINSTER, N.J., Jan. 10, 2019 (GLOBE NEWSWIRE) — Matinas BioPharma Holdings, Inc. (NYSE AMER: MTNB), a clinical stage biopharmaceutical company, today announced they have signed an agreement with an undisclosed top global pharmaceutical company aimed to evaluate synergistic effects of Matinas’ lipid-nano-crystal (“LNC”) platform delivery technology with their partner’s nucleic acid polymer […]
Perflow Medical Expands IP Coverage for Novel Stream™ Dynamic Neuro-Thrombectomy Net Device and Cascade™ Non-Occlusive Remodeling Net
TEL AVIV, Israel, Jan. 10, 2019 /PRNewswire/ — Perflow Medical, an Israeli-based medtech company focused on next-generation neuro-interventional devices, today announced the issuance of four international patents that expand the global intellectual property (IP) coverage for the novel Stream™ Dynamic Neuro-Thrombectomy Net and Cascade™ Non-Occlusive Remodeling Net. Perflow’s growing global portfolio of eight issued […]
Boston Scientific Announces Preliminary Unaudited Sales for the Fourth Quarter and Full Year 2018
MARLBOROUGH, Mass., Jan. 8, 2019 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) generated sales, based upon preliminary unaudited financial information, of approximately $2.56 billion during the fourth quarter of 2018. This represents growth of approximately 6.3 percent on a reported basis, approximately 8.2 percent on an operational1 basis and approximately 7.0 percent on an organic2 basis, […]
Amgen Makes All Repatha® (evolocumab) Device Options Available In The US At A 60 Percent Reduced List Price
THOUSAND OAKS, Calif., Jan. 7, 2019 /PRNewswire/ — Amgen (NASDAQ: AMGN) today announced that as part of the Company’s commitment to improve patient affordability for an innovative biologic medicine for people with high cholesterol who are at risk for heart attacks and strokes, all Repatha® (evolocumab) device options, including the Pre-Filled Syringe and Pushtronex® (on-body infusor […]
AtriCure Reports Preliminary Results for Fourth Quarter and Full Year 2018
MASON, Ohio–(BUSINESS WIRE)–AtriCure, Inc. (Nasdaq: ATRC), a leading innovator in treatments for atrial fibrillation (Afib) and left atrial appendage (LAA) management, today announced preliminary financial results for the fourth quarter and full year 2018 and provided 2019 financial guidance. Preliminary, unaudited revenue for fourth quarter 2018 is expected to be […]
Intact Vascular Announces Launch of the Tack Endovascular System® in the EU and First Commercial Use in Germany
WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the first commercial use of its Tack Endovascular System® in multiple hospitals within Germany. A novel therapy for dissection repair following balloon angioplasty, the Tack® implant is a first-of-its kind device for patients with peripheral […]