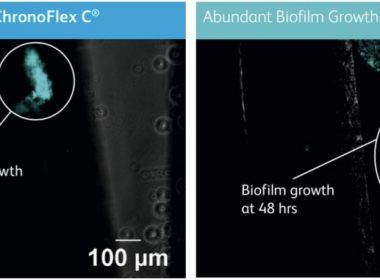
SAN DIEGO, Jan. 7, 2019 /PRNewswire/ — Access Scientific, LLC today announced publication, in the journal Medical Devices: Evidence & Research, of a groundbreaking study proving the superiority of POWERWAND™ catheter material, Chronoflex®C with BioGUARD™ (as compared with the standard polyurethane) with respect to bacterial attachment and biofilm formation. Utilizing state-of-the-art fluorescent microscopy, the […]
Other News
Shockwave Announces Appointment of Ray Larkin as Chairman of the Board
SANTA CLARA, Calif., Jan. 04, 2019 (GLOBE NEWSWIRE) — Shockwave Medical (“Shockwave”), a pioneer in the development and commercialization of intravascular lithotripsy to treat complex calcified cardiovascular disease, today announced that its Board of Directors has appointed Ray Larkin Jr. to join its Board of Directors and to serve as […]
Esperion Announces Agreement with Daiichi Sankyo Europe (DSE) to Commercialize Bempedoic Acid in Europe
ANN ARBOR, Mich., Jan. 04, 2019 (GLOBE NEWSWIRE) — Esperion Therapeutics (NASDAQ: ESPR) today announced that they have entered into a licensing agreement with Daiichi Sankyo Europe (DSE) providing DSE with exclusive rights to commercialize bempedoic acid and the bempedoic acid / ezetimibe combination pill in the European Economic Area […]
Leigh Elkolli Promoted to Chief Financial Officer at REVA Medical
SAN DIEGO, Jan. 03, 2019 (GLOBE NEWSWIRE) — REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”), a leader in bioresorbable polymer technologies for vascular applications, announced the promotion of Leigh Elkolli to the position of Chief Financial Officer and Corporate Secretary, effective January 4, 2019. She will replace Brandi […]
MRI Interventions Receives FDA Clearance for ClearPoint® PURSUIT™ Neuro Aspiration System
IRVINE, Calif., Jan. 03, 2019 (GLOBE NEWSWIRE) — MRI Interventions, Inc. (OTCQB:MRIC), a platform neurosurgery company with products designed for navigation in procedures involving deep-brain stimulation, ablation, and gene and drug delivery today announced FDA Clearance of the ClearPoint PURSUIT Neuro Aspiration system. The PURSUIT device was designed in collaboration […]
AngioDynamics Reports Fiscal 2019 Second Quarter Financial Results
LATHAM, N.Y.–(BUSINESS WIRE)–AngioDynamics, Inc. (NASDAQ:ANGO), a leading provider of innovative, minimally invasive medical devices for vascular access, peripheral vascular disease, and oncology, today announced financial results for the second quarter of fiscal year 2019, which ended November 30, 2018. “We are very pleased with our second quarter financial results, which are marked by growth […]
Endologix Takes Decisive Action to Optimize Patient Outcomes by Ensuring Nellix System Used Only within Current Indications
IRVINE, Calif.–(BUSINESS WIRE)–Endologix® Inc. (Nasdaq: ELGX) (“Endologix” or the “Company”), a developer and marketer of innovative treatments for aortic disorders, announced today that in order to ensure optimal outcomes for patients, unrestricted sales and use of the Nellix System will cease immediately, and the product will only be available for […]
Neovasc Receives Nasdaq Notification Regarding Minimum Market Value Deficiency
VANCOUVER, Jan. 3, 2019 /PRNewswire/ – (“Neovasc” or the “Company”) (NASDAQ: NVCN)(TSX: NVCN), announced today that it has received written notification (the “Notification Letter”) from The Nasdaq Stock Market LLC (“Nasdaq”) notifying the Company that it is not in compliance with the minimum market value requirement set forth in Nasdaq Rules for continued listing on the Nasdaq […]
Merit Medical Announces Appointment of Two New Directors
SOUTH JORDAN, Utah, Jan. 03, 2019 (GLOBE NEWSWIRE) — Merit Medical Systems, Inc. (NASDAQ: MMSI), a leading manufacturer and marketer of proprietary disposable devices used in interventional, diagnostic and therapeutic procedures, particularly in cardiology, radiology, oncology, critical care and endoscopy, today announced that its Board of Directors increased the number […]
Hemostemix Announces Two Additional Sites Enrolled in Phase II Clinical Trial
CALGARY, Alberta, Jan. 03, 2019 (GLOBE NEWSWIRE) — Hemostemix Inc. (“Hemostemix” or the “Company”) (TSX VENTURE: HEM; OTCQB:HMTXF) is pleased to announce that it has received all approvals from two new medical centers to be clinical trial sites for the Company’s Phase II clinical trial for critical limb ischemia (“CLI”). […]