BILLDAL, Sweden, March 7, 2017 /PRNewswire/ — Unfors RaySafe has introduced the RaySafe i3, to its suite of real-time dosimetry products, at the European Society of Radiology in Vienna. The RaySafe Real-Time Dosimetry solution, introduced in 2012, helps physicians and clinical staff visualise X-ray exposure in real time using easy-to-read bar […]
Other News
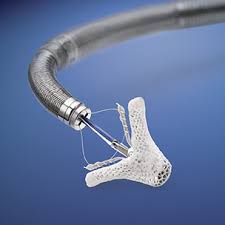
Late-Breaking Data on Abbott’s MitraClip® System Show Continued Benefit for People with Mitral Regurgitation, Most Common Heart Valve Disease
WASHINGTON, March 18, 2017 /PRNewswire/ — Abbott (ABT) today announced favorable one-year outcomes from the largest study of real-world experience for the MitraClip system in transcatheter mitral valve repair (TMVR) procedures in the United States. MitraClip treats people with degenerative mitral regurgitation (DMR, also known as leaky heart valve), a […]
Toshiba Medical Systems’ New Premium Cardiovascular Ultrasound Wins FDA Approval
Toshiba Medical’s New Premium Cardiovascular Ultrasound Receives FDA Clearance The Aplio i900 Delivers Fast, High Quality Images for Better Diagnostic Confidence TUSTIN, Calif.–(BUSINESS WIRE)–Cardiologists can now access the advanced ultrasound imaging technology needed for fast and confident diagnoses with Toshiba Medical’s Aplio™ i900. The newly FDA-cleared system is the latest […]
BioSig Technologies Announces Strategic Partnership with Mayo Clinic
Ten-year agreement provides know-how, intellectual property development and clinical resources to commercialize the PURE EP System and develop future technologies Minneapolis, MN, March 17, 2017 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (OTCQB: BSGM), a medical device company developing the PURE EP(TM) System, a proprietary platform designed to address an unmet technology need […]
Perflow Medical Announces Issuance of Patent for The Stream™ Dynamic Neuro-Thrombectomy Net to Improve Thrombus Removal Post-Stroke
NEWS PROVIDED BY Perflow Medical 07 Dec, 2016, 08:05 ET SHARE THIS ARTICLE TEL AVIV, Israel, Dec. 7, 2016 /PRNewswire/ — Perflow Medical, an Israeli-based medtech company focused on next-generation neurointerventional devices, today announced the issuance of U.S. Patent No. 9510855, by the U.S. Patent and Trademark Office (USPTO), for […]
Clinical study validates efficiencies of Stereotaxis Niobe ES system compared to Niobe II system
ST. LOUIS, March 14, 2017 (GLOBE NEWSWIRE) — Stereotaxis, Inc. (OTCQX:STXS), a global leader in innovative robotic technologies for the treatment of cardiac arrhythmias, today reported results of a study conducted at Centre Hospitalier Universitaire (CHU) of Saint-Étienne, France, which validates the advantages of the Niobe ® ES magnetic navigation […]
Innovative Cardiovascular Solutions Announces Successful First Cases in European Clinical Study of EMBLOK Embolic Protection System
NEWS PROVIDED BY Innovative Cardiovascular Solutions 15 Mar, 2017, 15:09 ET SHARE THIS ARTICLE MILAN, March 15, 2017 /PRNewswire/ — Innovative Cardiovascular Solutions (ICS), a privately held medical device company pioneering novel solutions for embolic protection during transcatheter aortic valve replacement (TAVR), announced today successful first European clinical cases using the […]
PQ Bypass Announces CE Mark for DETOUR Percutaneous Bypass Technologies
PQ Bypass Announces CE Mark for DETOUR Percutaneous Bypass Technologies Trio of proprietary technologies expands treatment options for patients with extremely long superficial femoral artery blockages due to peripheral artery disease March 13, 2017 08:00 AM Eastern Daylight Time SUNNYVALE, Calif.–(BUSINESS WIRE)–PQ Bypass, today announced CE (Conformité Européenne) Mark approval […]
Stryker Announces an Early Stop to Enrollment in the DAWN™ Trial Due to the High Probability of Trial Success
KALAMAZOO, MICHIGAN (PRWEB) MARCH 08, 2017 Stryker announced an early end to patient enrollment in the DAWN Trial, a clinical study designed to compare mechanical thrombectomy with the Trevo® Retriever plus medical therapy against medical therapy alone when initiated within six to 24 hours after time last known well. The […]
AtriCure Names National Principal Investigator for the CONVERGE IDE Clinical Trial
MASON, Ohio–(BUSINESS WIRE)–AtriCure, Inc. (Nasdaq: ATRC), a leading innovator in surgical treatments for atrial fibrillation (Afib) and left atrial appendage management, today announced that it has named Dr. David B. DeLurgio as the national principal investigator (PI) for the CONVERGE IDE clinical trial. “I am honored and humbled to be […]