BOULDER, Colo.–(BUSINESS WIRE)–Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced that the Company will present data on EDG-7500 at the American College of Cardiology’s Annual Scientific Session (ACC.24). EDG-7500 is a first-in-class oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with HCM and other diseases of diastolic dysfunc
Other News
Milestone Pharmaceuticals Announces Resubmission of New Drug Application for Etripamil for Treatment in Paroxysmal Supraventricular Tachycardia
MONTREAL and CHARLOTTE, N.C., March 28, 2024 (GLOBE NEWSWIRE) — Milestone® Pharmaceuticals Inc. (Nasdaq: MIST), a biopharmaceutical company focused on the development and commercialization of innovative cardiovascular medicines, today announced the resubmission of its New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for etripamil, the Company’s lead investigational product for the management of paroxysmal supraventricular tachycardia (PSVT).
Polymedco announces US FDA clearance of the PATHFAST high-sensitivity cardiac troponin I (hs-cTnI-II) test as an aid in the diagnosis of myocardial infarction
PATHFAST hs-cTnI-II becomes first and only hs-cTn test cleared for point-of-care use in the United States, delivering results up to three times faster than core lab testing CORTLANDT MANOR, N.Y., March 27, 2024 /PRNewswire/ — The PATHFAST hs-cTnI-II, a breakthrough high-sensitivity…
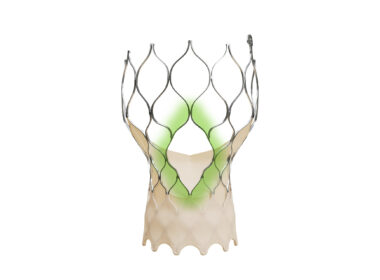
Medtronic announces FDA approval of newest-generation Evolut TAVR system for treatment of symptomatic severe aortic stenosis
The Evolut™ FX+ TAVR system leverages market-leading valve performance with addition of larger windows to facilitate coronary access DUBLIN, March 27, 2024 /PRNewswire/ — Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced that the United States Food and…
LeMaitre to Present at the 23rd Annual Needham Virtual Healthcare Conference
BURLINGTON, Mass., March 26, 2024 (GLOBE NEWSWIRE) — LeMaitre Vascular, Inc. (Nasdaq:LMAT) announced today that JJ Pellegrino, Chief Financial Officer, will present virtually at the 23rd Annual Needham Virtual Healthcare Conference on Wednesday, April 10, 2024, at 11:30 AM ET.
Adagio Medical Announces CE Mark approval of VT Cryoablation System, Plans for Immediate Commercialization in Select European Centers
LAGUNA HILLS, Calif., March 25, 2024 /PRNewswire/ — Adagio Medical, Inc., a leading innovator in catheter ablation technologies for ventricular tachycardia (“VT”) and atrial fibrillation (“AF”), today announced CE Mark approval of its ultra-low temperature cryoablation (“ULTC”) system for the treatment of monomorphic ventricular tachycardia. The system consists of the upgraded cryoablation console, […]
HeartFlow Initiates DECIDE Registry to Evaluate Utility of HeartFlow AI-Enabled Plaque Analysis for Patients with Suspected Coronary Artery Disease
The largest prospective registry of its kind will build upon clinical data supporting the use of plaque insights to empower clinical decision making for patients with suspected coronary artery disease The largest prospective registry of its kind will build upon clinical data supporting the use of plaque insights to empower clinical decision making for patients with suspected coronary artery disease
Texas Cardiac Arrhythmia Institute at St. David’s Medical Center to host international conference on complex cardiac arrhythmias
EPLive 2024 is a two-day conference that draws the world’s top cardiac electrophysiology experts AUSTIN, Texas, March 26, 2024 /PRNewswire/ — On April 18 and 19, 2024, the Texas Cardiac Arrhythmia Institute (TCAI) at St. David’s Medical Center will host its seventh international…
AbbaDox’s RIS Implementation at Lake Medical Imaging Sets a New Industry Standard in Radiology Information Systems
As the healthcare industry faces the phase-out of a major Radiology Information System (RIS) provider, AbbaDox steps up as the superior alternative, showcasing unparalleled efficiency in migrating Lake Medical Imaging to its advanced RIS & Patient Engagement platform. THE VILLAGES, Fla.,…
Cleerly® ISCHEMIA™ Demonstrates Robust Diagnostic Accuracy and Prognostic Utility in Analyses from Large Scale Clinical Trials
DENVER–(BUSINESS WIRE)–Cleerly, the company on a mission to create a new standard of care to aid in the diagnosis of heart disease, shared findings from a study published online in the Journal of the American College of Cardiology: Cardiovascular Imaging on March 13, 2024. The study describes the validation of Cleerly’s artificial intelligence-guided quantitative coronary CT angiography (AI-QCT) ISCHEMIA technology for diagnostic accuracy and prognostic risk stratification. In two trials,1-2