SAN DIEGO–(BUSINESS WIRE)–Vektor Medical, a pioneer in non-invasive, AI-based arrhythmia analysis technology, today announced a $16 million Series A investment co-led by Solas BioVentures and TVM Capital Life Science. This funding underscores investor confidence in Vektor’s vision and ability to successfully execute its mission to revolutionize arrhythmia care. “The medical […]
Other News
Houston-Based resTOR Longevity Clinic to Offer Cardio Diagnostics’ AI-Driven Epigenetic-Genetic Heart Disease Tests
CHICAGO–(BUSINESS WIRE)–Cardio Diagnostics Holdings, Inc. (NASDAQ: CDIO), an AI-driven precision cardiovascular medicine company, announces that Houston-based resTOR Longevity Clinic will integrate Cardio Diagnostics’ solutions into its battery of tests for new patients in its longevity-focused concierge clinic. This collaboration marks a significant milestone for Cardio Diagnostics, as resTOR will be the […]
LivaNova Announces Vladimir A. Makatsaria as CEO and Board Director
LONDON–(BUSINESS WIRE)–LivaNova PLC (Nasdaq: LIVN), a market-leading medical technology company, today announced its Board of Directors named Vladimir A. Makatsaria as the Company’s Chief Executive Officer (CEO) and a member of the Board of Directors, effective March 1, 2024. Makatsaria most recently served as Company Group Chairman at Johnson & […]
Acarix CADScor System Now Listed on U.S. VA Federal Supply Schedule
NEW YORK, Feb. 5, 2024 /PRNewswire/ — Acarix, a leader in advanced acoustic-based cardiac diagnostic devices today announces the CADScor® System has been added to the United States Federal Supply Schedule (FSS). The nationwide contract simplifies purchasing for the Veterans Health…
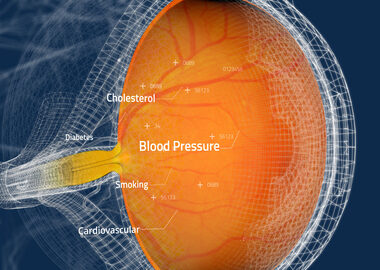
Toku Obtains CE and UKCA Marks for AI Cardiovascular Risk Assessments Through the Eye
SAN DIEGO–(BUSINESS WIRE)–Toku, Inc., a commercial medical device company specializing in AI assessment of retinal images, announced today that it has obtained CE and UKCA Marks for its patented CLAiR technology. This significant milestone underscores Toku’s commitment to maintaining the highest standards of quality and safety and allows access to […]
8 Best WordPress Training Courses for Beginners in 2024
WordPress is a powerful content management system (CMS) that pretty much anyone can use to make a stunning, custom website. You don’t even have to know how to code to use it. But, if you’ve never used the platform before, there’s a bit of a learning curve. If that’s exactly […]
8 Best WordPress Training Courses for Beginners in 2024
WordPress is a powerful content management system (CMS) that pretty much anyone can use to make a stunning, custom website. But, if you’ve never used the platform before, there’s a bit of a learning curve. In this post, we’re going to share eight of the best WordPress training courses for […]
Boston Scientific Receives FDA Approval for FARAPULSE™ Pulsed Field Ablation System
MARLBOROUGH, Mass., Jan. 31, 2024 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) announced it has received U.S. Food and Drug Administration (FDA) approval for the FARAPULSE™ Pulsed Field Ablation (PFA) System. The FARAPULSE PFA System is indicated for the isolation of pulmonary veins in the treatment of drug-refractory, recurrent, symptomatic, paroxysmal (i.e., […]
Edwards’ EVOQUE Valve Replacement System First Transcatheter Therapy to Earn FDA Approval for Tricuspid Valve
IRVINE, Calif.–(BUSINESS WIRE)– Edwards Lifesciences Corporation (NYSE: EW) today announced the company’s EVOQUE tricuspid valve replacement system is the first transcatheter therapy to receive U.S. Food and Drug Administration (FDA) approval for the treatment of tricuspid regurgitation (TR). The EVOQUE system is indicated for the improvement of health status in patients […]
Complete Tutorial: How to Build a Membership Site on WordPress
Creating a membership site on WordPress can be valuable to your new or existing business. The site may serve to offer online courses and exclusive content or simply to build an online community. Regardless of your budget, you can build a membership site without spending too much. All you need […]