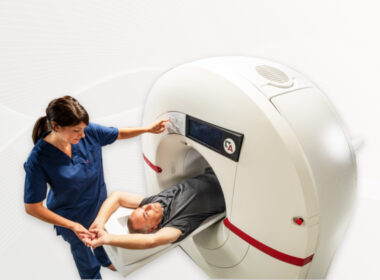
Amsterdam, the Netherlands and Chicago, USA – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today showcases the world’s first mobile MRI system with helium-free operations at #RSNA23. BlueSeal MR Mobile [1], the industry’s first and only 1.5T fully sealed magnet, delivers patient-centric MRI services where […]
Other News
Konica Minolta Healthcare Showcases New Radiography and Ultrasound Solutions at RSNA 2023 That Help Clinicians See What Matters Most
The company celebrates 150 years of imaging innovation and leading through change Universal DR Long Detector Panel At RSNA 2023, Konica Minolta Healthcare launches its newest flat panel detector, the Universal DR 17 x 48 Long Detector, an advanced diagnostic solution to meet the needs of musculoskeletal, orthopedic, and neuro […]
Cloud-Based Automated Revenue Collection and Prior-Authorization Workflow Solutions for Exa Platform Launched at RSNA 2023
At RSNA 2023, Konica Minolta Healthcare introduces Exa Connect and Exa Clear Authorization as part of the Exa® Platform. WAYNE, N.J., Nov. 26, 2023 (GLOBE NEWSWIRE) — Approximately half of recommended patient follow-up contained in radiology reports, including incidental findings, are not acted upon and often result in missed […]
Brainomix Targets US Expansion with the Launch of its Cutting-Edge Stroke AI Platform Following Series of FDA Clearances
Launched at the Society of Vascular and Interventional Neurology (SVIN) Conference in Miami, the Brainomix 360 platform is powered by best-in-class algorithms, delivering a unique technology for both specialist and non-specialist stroke centers that is poised to transform stroke care…
Acutus Medical Announces Strategic Realignment of Resources and Corporate Restructuring
CARLSBAD, Calif., Nov. 08, 2023 (GLOBE NEWSWIRE) — Acutus Medical, Inc. (“Acutus” or the “Company”) (Nasdaq: AFIB), today announced a realignment of resources and corporate restructuring. Scott Huennekens, Chairman of Acutus, commented, “Following an extensive strategic review by the Company’s Board of Directors, we are taking the hard but necessary […]
SUPIRA MEDICAL, INC., A SHIFAMED PORTFOLIO COMPANY, RECEIVES FDA BREAKTHROUGH DEVICE DESIGNATION AS THE COMPANY CLOSED $40M IN SERIES D FINANCING
LOS GATOS, Calif., Nov. 21, 2023 /PRNewswire/ — Supira Medical, Inc., a Shifamed portfolio company, announced today that it has received U.S. Food and Drug Administration (FDA) breakthrough device designation for its Supira System, a next-generation percutaneous ventricular assist device (pVAD). This approval comes as the company closed $40M in Series D financing and prepares […]
Arineta Receives FDA Clearance for its Deep Learning Image Reconstruction Technology in SpotLight™ Cardiovascular CT Scanners
Caesarea, Israel – November 20, 2023 – Arineta Cardio Imaging, a leader in point-of-care cardiovascular CT solutions, today announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its deep learning image reconstruction (DLIR) technology for use in its SpotLight™ family of cardiovascular CT (CCT) scanners. This next-generation […]
InsiteOne is Acquiring BRIT Systems Cloud-Native RIS/PACS/VNA
InsiteOne will continue to develop and support BRIT Systems RIS/PACS while building new solutions that address key needs in radiology and enterprise workflows. WALLINGFORD, Conn., Nov. 21, 2023 /PRNewswire/ — InsiteOne, an original founder of true cloud-based Vendor Neutral Archive (VNA)…
Cardio Diagnostics Holdings, Inc. Announces Breakthrough in Coronary Heart Disease Detection with PrecisionCHD Test, Published in the Journal of the American Heart Association
CHICAGO–(BUSINESS WIRE)–Cardio Diagnostics today announced the publication of its groundbreaking study in the Journal of the American Heart Association (JAHA),
Humacyte Announces Two Presentations at the VEITHsymposium® of Positive Clinical Results of the Human Acellular Vessel™ (HAV™) in the Treatment of Vascular Trauma
DURHAM, N.C., Nov. 17, 2023 (GLOBE NEWSWIRE) — Humacyte, Inc. (Nasdaq: HUMA), a clinical-stage biotechnology platform company developing universally implantable, bioengineered human tissue at commercial scale, today announced the presentation of positive V005 Phase 2/3 clinical trial results of the investigational Human Acellular Vessel (HAV) in the treatment of vascular trauma, […]