MIAMI LAKES, Fla., Oct. 24, 2023 /PRNewswire/ — Cordis, a worldwide leader in the development and manufacturing of interventional cardiovascular and endovascular technology, has announced completion of patient enrollment in SUCCESS PTA, its large post-market study with the SELUTION SLR™…
Other News
Pulse Biosciences Appoints Dr. Niv Ad as Chief Science Officer, Cardiac Surgery
HAYWARD, Calif.–(BUSINESS WIRE)–Pulse Biosciences, Inc. (Nasdaq: PLSE, “the Company”), a company primarily focused on leveraging its novel and proprietary Nanosecond Pulsed Field Ablation (nsPFA) technology for the treatment of atrial fibrillation, today announced the appointment of Niv Ad, M.D. as Chief Science Officer, Cardiac Surgery. “After working with the Pulse Biosciences’ team and participating in preclinical studies using the nsPFA clamp for cardiac ablation, it was immediately clear
Texas County Memorial Hospital Goes Live with RapidAI to Enhance Neurovascular Decision Making and Rural Stroke Care
SAN MATEO, Calif. & HOUSTON, Mo.–(BUSINESS WIRE)–RapidAI, a global leader in developing Artificial Intelligence (AI) and technology workflow solutions to combat life-threatening neurovascular, cardiac and vascular diseases, is pleased to announce Texas County Memorial Hospital’s (TCMH) implementation of RapidAI’s core stroke imaging and workflow products – including Rapid ICH, LVO, CTA and CTP – as part of its ongoing commitment to enhancing the quality of stroke care for patients in rural so
Aktiia SA collaborates on landmark study led by McMaster University, the Private University of Liechtenstein, and the Dr. Risch Laboratory for the determination of cardiovascular risk factors on more than 2000 people
NEUCHATEL, Switzerland, Oct. 24, 2023 /PRNewswire/ — Aktiia SA, the world leader in cuffless blood pressure technology, is proud to announce its collaboration with the Genetic and phenotypic determinants of blood pressure and other cardiovascular risk factors (GAPP) study investigators,…
Frontier Bio Leads Medical Innovation with “Lab-Grown” Blood Vessel Technology for Replacing Animal Testing
SAN FRANCISCO, Oct. 24, 2023 /PRNewswire/ — Frontier Bio Corporation has unveiled a transformative method designed to fabricate living human blood vessels, setting the stage for a radical shift in medical device testing. At present, vascular medical devices must be assessed for safety…
EBR Systems nominated for MTAA Excellence in Medical Technology award
SUNNYVALE, Calif., Oct. 23, 2023 /PRNewswire/ — EBR Systems, Inc. (ASX:EBR), the developer of the world’s only wireless cardiac pacing device for heart failure, is pleased to announce it has been named as a nominee for the 2023 Medical Technology Association of Australia (MTAA) Kerrin Rennie award. This nomination by the MTAA recognizes the potential of EBR Systems’ […]
Genesis MedTech initiates enrolment in its North American Early Feasibility Study using the J-Valve™ Transfemoral System for patients with severe aortic regurgitation
BURLINGAME, Calif., Oct. 23, 2023 /PRNewswire/ — Genesis MedTech, a leading medical device company, today announced initiation of enrolment in its North American Early Feasibility Study using its dedicated TAVR system, J-Valve™ Transfemoral (TF) System. The procedure was successfully performed by Dr. Dean Kereiakes, MD, Dr. Santiago Garcia, MD, and the team at The Lindner […]
Mindray Expands Its Cardiac Biomarker Portfolio with Cutting-Edge hs-cTnI and NT-proBNP Assays, Empowering Enhanced Cardiovascular Diseases Care
SHENZHEN, China, Oct. 23, 2023 /PRNewswire/ — Mindray (SZSE: 300760), a global leader in medical devices and solutions, introduces high-sensitivity troponin I (hs-cTnI) and NT-proBNP cardiac biomarkers. The additions have enhanced Mindray’s diverse portfolio of cardiac biomarkers for cardiovascular diseases (CVDs) diagnosis and management. While CVDs are the leading cause of death […]
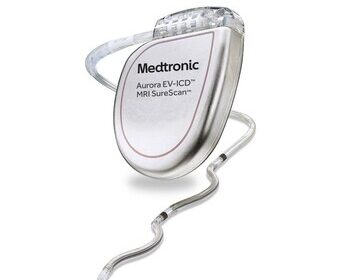
Medtronic receives FDA approval for extravascular defibrillator to treat abnormal heart rhythms, sudden cardiac arrest
DUBLIN, Oct. 23, 2023 /PRNewswire/ — Medtronic plc (NYSE:MDT), a global leader in healthcare technology, has received U.S. Food and Drug Administration (FDA) approval for the Aurora EV-ICD™ MRI SureScan™ (Extravascular Implantable Cardioverter-Defibrillator) and Epsila EV™ MRI SureScan™ defibrillation lead to treat dangerously fast heart rhythms that can lead to sudden cardiac […]
Advanced Bifurcation Systems Inc. Receives FDA Breakthrough Device Designation
LIVERMORE, Calif., Oct. 23, 2023 /PRNewswire/ — Advanced Bifurcation Systems Inc. (ABS), a pioneer in comprehensive solutions for bifurcation lesions in coronary angioplasty, today announced that it has received the Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for its novel coronary artery bifurcation stenting technology. This prestigious designation […]