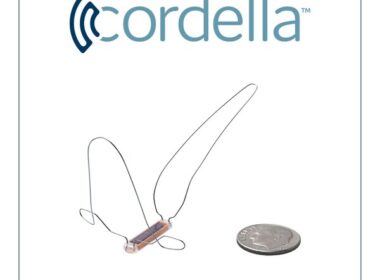
NAPERVILLE, Ill., Oct. 9, 2023 /PRNewswire/ — Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure (HF), announced it has received Investigational Device Exemption (IDE) approval from the FDA for a subsequent multicenter study, PROACTIVE-HF 2, which will evaluate the company’s Cordella Sensor for pulmonary artery […]
Other News
Late-Breaking Data Confirms Safety of Axon Therapies’ Innovative Heart Failure Procedure and Identifies Patients Most Likely to Benefit from SAVM Therapy
SANTA CLARA, Calif., Oct. 8, 2023 /PRNewswire/ — Axon Therapies, a private company focused on addressing a root cause of heart failure, today announced 6-month results of the REBALANCE-HF randomized, blinded feasibility trial of the Splanchnic Ablation for Volume Management (SAVM) procedure for heart failure with preserved ejection fraction (HFpEF). The main objectives of the […]
Rejuvenate Bio Announces Preclinical Data for Gene Therapy Candidate RJB-0402 for the Treatment of Arrhythmogenic Cardiomyopathy
SAN DIEGO–(BUSINESS WIRE)–Rejuvenate Bio, today announced new preclinical efficacy data for RJB-0402, a gene therapy targeting key disease-mediating pathways. RJB-0402 demonstrated efficacy in a mouse model of arrhythmogenic cardiomyopathy (ACM), an inherited disease caused by mutations in one of several genes encoding desmosomal proteins. A single dose of RJB-0402 was […]
CONTEGO MEDICAL ANNOUNCES START OF ENROLLMENT IN PERFORMANCE III DIRECT TRANSCAROTID ACCESS STENTING TRIAL
RALEIGH, N.C., Oct. 6, 2023 /PRNewswire/ — Contego Medical Inc., a company dedicated to improving patient outcomes and procedural efficiency in the treatment of carotid and peripheral vascular disease, today announced enrollment of the first patient in the PERFORMANCE III Trial. PERFORMANCE III is a prospective, multicenter trial aimed at further evaluating […]
Marizyme, Inc. Announces FDA Clearance for Flagship Product, DuraGraft™
JUPITER, FL, Oct. 06, 2023 (GLOBE NEWSWIRE) — via NewMediaWire – Marizyme, Inc. (OTCQB:MRZM) (“Marizyme” or the “Company”), a global medical technology company focused on the development of products to address unmet clinical needs, today announced that it was granted a de novo from the U.S. Food and Drug Administration (FDA) for its […]
LivaNova Board Appoints J. Christopher Barry as New Director
LONDON–(BUSINESS WIRE)–LivaNova PLC (Nasdaq: LIVN), a market-leading medical technology company, announced its Board of Directors has appointed J. Christopher Barry to the Board, effective today. Barry will serve on the Audit and Compliance Committee. Andrea Saia, who has served as a Director since 2016, will retire from the LivaNova Board […]
Biosense Webster Study Links Lower Risk of Heart Failure in Atrial Fibrillation Patients Treated with Catheter Ablation Compared to Antiarrhythmic Drugs
Irvine, CA October 5, 2023 – Biosense Webster, Inc. the global leader in cardiac arrhythmia treatment and part of Johnson & Johnson MedTech,1 announced today new data from a Biosense Webster funded study which sought to compare the risk of heart failure incidence among atrial fibrillation (AFib) patients treated with […]
MedAxiom Recruits Test Sites for Peer Review Plus, World’s First Crowdsourced Clinical Performance and Case Evaluation Solution
NEPTUNE BEACH, Fla.–(BUSINESS WIRE)–MedAxiom, the cardiovascular community’s premier destination for organizational performance solutions, has developed Peer Review Plus™, the world’s first clinical peer review solution to effectively and efficiently conduct unbiased performance evaluations and case reviews with input from a nationwide network of peers. MedAxiom is currently recruiting healthcare organizations […]
Elixir Medical To Present Randomized Clinical Dataset Featuring World’s First Triple Drug-Eluting Coronary Implant Eluting Two Anticoagulants and Sirolimus at TCT 2023
MILPITAS, Calif.–(BUSINESS WIRE)–Elixir Medical, a developer of innovative cardiovascular technologies, announced it will present primary endpoint and 6-month data from its DESyne BDS Plus Randomized Clinical Trial (RCT), a prospective, multicenter, single-blind study, as part of The Innovation Program at the Transcatheter Cardiovascular Therapeutics (TCT) conference in San Francisco. The data […]
Millar to Unveil Revolutionary Chronic Pressure Measurement Platform at MD&M Minneapolis
HOUSTON, Oct. 5, 2023 /PRNewswire/ — Millar, OEM solutions partner and leader in MEMS pressure sensor integration, is thrilled to announce that it will officially unveil its groundbreaking chronic pressure measurement technology platform, TiSense, at the upcoming MD&M tradeshow in Minneapolis on October 10-11, 2023. This event serves as the perfect stage for Millar to introduce TiSense […]