Earlier and more precise detection of cardiac amyloidosis is critical: once considered rare and overlooked, it is now moving into the mainstream of cardiology and public awareness, with new drugs, national campaigns and data demonstrating survival benefits that are enhanced when patients…
Other News
American Medical Association Published CPT Code for Caristo’s Coronary Inflammation Detection Technology
AMA assigns CPT codes 0992T and 0993T to Caristo’s CaRi-Heart® AI-powered analysis of perivascular fat and cardiovascular risk, which are included in the 2026 CPT® Codebook New CPT codes mark a major step toward making CaRi-Heart technology available in everyday clinical care in the U.S….
Heartflow Announces FDA 510(k) Clearance and Launch of Next Generation Heartflow Plaque Analysis Platform
AI-Powered Heartflow Plaque Analysis to be Covered by Cigna Health Plans Nationwide
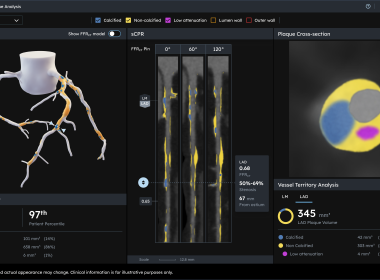
Next Generation Heartflow Plaque Analysis
Our Next Generation Plaque Analysis platform harnesses an FDA-cleared updated algorithm, vastly expanded nomogram, and advanced 3D color-coded visualization to deliver unprecedented insight into plaque type, volume, and distribution. This update marks the most sophisticated iteration of Heartflow’s technology – empowering clinicians to make confident care decisions with ease.
MOUNTAIN VIEW, Calif., Sept. 22, 2025 (GLOBE NEWSWIRE) — Heartflow, Inc. (Heartflow) (Nasdaq: HTFL), the leader in AI technology for coronary artery disease (CAD), today announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Next Gen Heartflow Plaque Analysis algorithm and the platform is now available. The technology features an updated algorithm, vastly expanded nomogram, and advanced 3D color-coded visualization of plaque type, volume, and distribution, empowering clinicians with the insights needed to make confident care decisions with ease. Heartflow also announced that Heartflow Plaque Analysis will be covered by Cigna across all of its lines of business, including Commercial and Medicare Advantage plans, beginning in October. Advanced Heartflow Plaque Analysis Platform Provides Clarity in 3D Heartflow Plaque Analysis is the only FDA-cleared, AI-powered plaque quantification tool with a reported 95% agreement with the gold standard, IVUS, using blinded core lab adjudication.1 The latest algorithm advancement shows 21% improvement in plaque detection compared to the first-generation algorithm, so clinicians can be confident in their diagnosis and management of CAD.2 “Understanding not only how much plaque is present, but also plaque type and distribution, is critical in predicting patient risk and guiding personalized treatment,” said Matthew Budoff, M.D., Professor of Medicine at the David Geffen School of Medicine at the University of California Los Angeles (UCLA) Medical Center. “With these enhanced capabilities, Heartflow Plaque Analysis provides clinicians with the clarity we need to move from detection to decision with speed and confidence.” Leveraging enhanced AI, this new Plaque Analysis update marks the most sophisticated iteration of Heartflow’s technology. It provides clinicians with an intuitive visual representation of plaque types, enabling rapid assessment of CAD location and severity. Heartflow’s enhanced nomogram is powered by data from ~273,000 patients—a dataset 9x larger than any current plaque quantification study.3 Cigna Adds Nationwide Coverage for Heartflow Plaque Analysis Cigna is the second national insurer to update its policies to cover Heartflow Plaque Analysis to fully align with the guidelines issued by radiology benefit manager EviCore, following a similar decision by UnitedHealthcare. The updated coverage will become effective on October 1, 2025 for Cigna patients with acute or stable chest pain and mild-to-moderate narrowing of coronary arteries (1-69% stenosis) identified on coronary CTA. “Heartflow is proud to lead the way in coronary plaque analysis with our Next Gen platform, delivering the most representative visualization and context available for assessing patients’ disease and risk. Cigna’s decision to cover Heartflow Plaque Analysis is a testament to the power of our technology to positively impact care for its members across the United States,” said John Farquhar, President and CEO of Heartflow. “Heartflow Analyses give clinicians a view of coronary plaque by type and impact on blood flow with FFRCT that’s key to informing management strategies. These latest advancements build on Heartflow’s record of proven innovation, leveraging clinical rigor and the world’s largest dataset of coronary CTA images to continually improve our technology for clinicians and patients.” Heartflow’s continued advancement of plaque analysis technology follows the company’s recent unveiling of the landmark DECIDE Registry data, which showed Heartflow Plaque Analysis led to medical management change in over 50% of patients beyond coronary CTA alone, resulting in an expected ~15% event reduction.4,5 Heartflow is dedicated to reshaping cardiovascular care and ensuring that physicians and patients have access to comprehensive, accurate, and efficient solutions for precision coronary care. About Heartflow, Inc.Heartflow is transforming coronary artery disease from the world’s leading cause of death into a condition that can be detected early, diagnosed accurately, and managed for life. The Heartflow One platform uses AI to turn coronary CTA images into personalized 3D models of the heart, providing clinicians with actionable insights into plaque volume and composition and its impact on blood flow – without the need for invasive procedures. Supported by ACC/AHA guidelines and more than 600 peer-reviewed publications, Heartflow has helped clinicians manage nearly 500,000 patients worldwide. Discover how we’re shaping the future of cardiovascular care at heartflow.com. 1 Ihdayhid A, et al. Radiol Cardiothorac Imaging. 2024. doi: 10.1148/ryct.230312 and internal bridging study with ICC correlation between first generation and second generation Plaque Analysis algorithm.2 U.S. Food and Drug Administration. 510(k) Premarket Notification: K250902. https://www.accessdata.fda.gov/cdrh_docs/pdf25/K250902.pdf3 Tzimas, et al., Presentation SCCT July 2025.4 DECIDE Registry. Rinehart, et al., Presented at SCCT July 2025.5 Collins et al. Lancet 2016. DOI: 10.1016/S0140-6736(16)31357-5 Media Contact Elliot Levy elevy@heartflow.com Investor Contact Nick Laudico nlaudico@heartflow.com A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/5f268838-f806-4612-995b-af3aa4676cc0
eMyosound’s Revolutionary Echocardiography Technology to start Clinical Trial in Three Boston Hospitals
New generation of echocardiography modality for myocardial characterization: Real-time myocardial 3D Shear Wave Elastography Boston, September 8, 2025 – eMyosound – a French medical device company focused on assessing cardiac diseases through its groundbreaking ultrasound technology – has announced it is beginning a clinical trial in three major Boston hospitals. Led […]
New Leaders Join Children’s Hospital of Philadelphia Cardiovascular Institute, Advancing Pediatric Research and Cardiac Care
Andrew Landstrom, MD, PhD, Appointed Inaugural Director of Translational Research at CHOP’s Cardiovascular Institute PHILADELPHIA, Sept. 22, 2025 /PRNewswire/ — Children’s Hospital of Philadelphia (CHOP) is pleased to announce the selection of Andrew Landstrom, MD, PhD, as the inaugural…
CRISPR Therapeutics and Sirius Therapeutics Announce First Patient Dosed in Phase 2 Trial of SRSD107 for Thromboembolic Disorders in Europe
ZUG, Switzerland and BOSTON and SAN DIEGO and SHANGHAI, Sept. 22, 2025 (GLOBE NEWSWIRE) — CRISPR Therapeutics (NASDAQ: CRSP), a biopharmaceutical company focused on creating transformative gene-based medicines for serious diseases, and Sirius Therapeutics, a clinical stage biotech company developing innovative small interfering RNA (siRNA) therapies for global markets, today announced that the first patient has been dosed in a Phase 2 clinical trial of SRSD107, a next-generation, long-acting Factor XI (FXI) siRNA for the prevention of venous thromboembolism (VTE) in patients undergoing total knee arthroplasty (TKA). SRSD107 is being co-developed by CRISPR Therapeutics and Sirius Therapeutics as part of a strategic collaboration to advance innovative treatments for cardiovascular and clotting-related diseases. “We are pleased to announce that our Phase 2 clinical trial is now underway, and the first patient has been dosed,” said Naimish Patel, M.D., Chief Medical Officer of CRISPR Therapeutics. “Until now, existing anticoagulant options have been limited by bleeding risk, frequent dosing, and complex management challenges for patients with high thrombotic risk. SRSD107 offers the potential to reduce pathological thrombosis while minimizing bleeding risk, with sustained but reversible pharmacodynamic effects and the possibility of infrequent dosing. We look forward to exploring how this differentiated approach could meaningfully improve outcomes for patients in need.” “We are very excited to announce that the first patient has been dosed in our Phase 2 trial of SRSD107, marking a significant milestone for this program,” said Patrick Yue, M.D., Chief Medical Officer of Sirius Therapeutics. “This study will evaluate clinical efficacy as proof of concept for Factor XI inhibition using our siRNA approach, building on the positive results from our Phase 1 trials, and we look forward to the progress of this trial.” The ongoing Phase 2 clinical trial is a randomized, multicenter, global study evaluating the safety and efficacy of SRSD107 for the prevention of VTE in patients undergoing TKA. The trial will assess the anticoagulant effects and pharmacological profile of SRSD107 and help inform dose selection for future pivotal studies, with the goal of confirming its potential as a differentiated approach for reducing thrombotic risk in patients. SRSD107 is designed to selectively inhibit FXI, a key driver of pathological thrombosis, with minimal impact on normal hemostasis. In prior Phase 1 clinical trials conducted in Australia and China, single doses of SRSD107 were well tolerated and demonstrated strong, sustained pharmacodynamic effects, including reductions of over 93% in FXI levels, along with more than a twofold increase in activated partial thromboplastin time (aPTT) relative to baseline. These effects were sustained, with responses maintained for up to six months post-dosing. SRSD107 has the potential to be a best-in-class FXI inhibitor, achieving deep reductions in FXI with the possibility of infrequent, semi-annual subcutaneous administration and offering reversibility not observed with other anti-FXI modalities. The addressable population includes patients with atrial fibrillation, VTE, cancer-associated thrombosis, chronic Coronary Artery Disease (CAD), chronic Peripheral Vascular Disease (PVD), end-stage renal disease requiring hemodialysis, and patients undergoing major orthopedic surgery, where bleeding risk limits existing therapies. About Thromboembolic DisordersThrombosis, or blood clot formation, is the common underlying mechanism of most cases of myocardial infarction, ischemic stroke, and venous thromboembolism. Published data in The Lancet1 estimate that thromboembolic disorders are estimated to account for approximately one in four deaths worldwide. About SRSD107SRSD107 is a novel double-stranded small interfering ribonucleic acid (siRNA), that is designed to target the human coagulation factor XI (FXI) mRNA and inhibit FXI protein expression. By modulating the intrinsic coagulation pathway, SRSD107 has the potential to provide anticoagulant and antithrombotic effects. About the CRISPR Therapeutics and Sirius Therapeutics CollaborationCRISPR Therapeutics and Sirius Therapeutics entered into a strategic collaboration in 2025 to develop and commercialize novel small interfering RNA (siRNA) therapies for thromboembolic disorders and other serious diseases. The lead program, SRSD107, is a long-acting siRNA targeting Factor XI (FXI) with the potential to offer best-in-class efficacy and safety. Under the agreement, the companies will co-develop SRSD107 and share costs and profits equally. CRISPR Therapeutics will lead commercialization in the U.S., while Sirius will lead in Greater China. The collaboration also provides CRISPR Therapeutics with the option to license up to two additional siRNA programs. This partnership expands CRISPR Therapeutics’ therapeutic portfolio into RNA-based medicines, complementing its ongoing efforts in gene editing and broadening its impact across serious and chronic diseases. For Sirius, the collaboration marks a major milestone in its mission to deliver innovative RNA-based therapies globally, leveraging deep expertise in siRNA design and delivery. About CRISPR TherapeuticsSince its inception over a decade ago, CRISPR Therapeutics has evolved from a research-stage company advancing gene editing programs into a leader that celebrated the historic approval of the first-ever CRISPR-based therapy. The Company has a diverse portfolio of product candidates across a broad range of disease areas including hemoglobinopathies, oncology, regenerative medicine, cardiovascular, autoimmune, and rare diseases. In 2018, CRISPR Therapeutics advanced the first-ever CRISPR/Cas9 gene-edited therapy into the clinic to investigate the treatment of sickle cell disease and transfusion-dependent beta thalassemia. Beginning in late 2023, CASGEVY® (exagamglogene autotemcel [exa-cel]) was approved in several countries to treat eligible patients with either of these conditions. The Nobel Prize-winning CRISPR technology has revolutionized biomedical research and represents a powerful, clinically validated approach with the potential to create a new class of potentially transformative medicines. To accelerate its efforts, CRISPR Therapeutics has formed strategic partnerships with leading companies, including Vertex Pharmaceuticals. CRISPR Therapeutics AG is headquartered in Zug, Switzerland, with its wholly-owned U.S. subsidiary, CRISPR Therapeutics, Inc., and R&D operations based in Boston, Massachusetts and San Francisco, California. To learn more, visit www.crisprtx.com. CRISPR THERAPEUTICS® standard character mark and design logo are trademarks and registered trademarks of CRISPR Therapeutics AG. CASGEVY® and the CASGEVY logo are registered trademarks of Vertex Pharmaceuticals Incorporated. All other trademarks and registered trademarks are the property of their respective owners. About Sirius TherapeuticsSirius is a global, clinical-stage biotech company developing innovative siRNA therapies focusing on the treatment of chronic diseases. The Company’s pipeline is centered around three key franchises with mega blockbuster potential: coagulation disorders, cardiometabolic diseases, and obesity. Sirius’ most advanced investigational programs include SRSD107, a FXI inhibitor targeting the anticoagulation market, SRSD216, an inhibitor of Lp(a) synthesis intended to address atherosclerotic cardiovascular disease, and SRSD384, an INHBE inhibitor for managing obesity. Founded in 2021 by a world-class leadership team and investors, Sirius has established an innovation center in the United States and a translational medicine center in China. Sirius has raised nearly US$150 million in funding to date from OrbiMed, Creacion Ventures, Hankang Capital, Delos Capital, and BioTrack Capital. CRISPR Therapeutics Forward-Looking StatementStatements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements made by Drs. Patel and Yue in this press release, as well as regarding any or all of the following: (i) CRISPR Therapeutics’ preclinical studies, clinical trials and pipeline products and programs, including, without limitation, expectations regarding data, safety and efficacy generally; (ii) data included in this press release, as well as the ability to use data from ongoing and planned clinical trials for the design and initiation of further clinical trials; (iii) the status and clinical progress of the SRSD107 clinical program and development timelines for such program; (iv) CRISPR Therapeutics’ strategy and goals; (v) the future activities of the parties pursuant to the collaboration and the expected benefits of CRISPR Therapeutics’ collaboration with Sirius Therapeutics; and (vi) the therapeutic value, development, and commercial potential of gene editing and delivery technologies and therapies, including CRISPR/Cas9. Risks that contribute to the uncertain nature of the forward-looking statements include, without limitation, the risks and uncertainties discussed under the heading “Risk Factors” in its most recent annual report on Form 10-K and in any other subsequent filings made by CRISPR Therapeutics with the U.S. Securities and Exchange Commission. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date they are made. CRISPR Therapeutics disclaims any obligation or undertaking to update or revise any forward-looking statements contained in this press release, other than to the extent required by law. This press release discusses investigational therapies and is not intended to convey conclusions about efficacy or safety as to those investigational therapies or uses of such investigational therapies. There is no guarantee that any investigational therapy will successfully complete clinical development or gain approval from applicable regulatory authorities. Sirius Therapeutics Forward Looking StatementThis press release may contain certain “forward-looking statements” which are not historical facts, but instead are predictions about future events based on Sirius Therapeutic’s current beliefs, assumptions and expectations, commonly identified by words such as “would”, “may”, “expects”, “believes”, “plans”, “intends”, “projects” and other terms with similar meaning. Although we believe that our predictions are reasonable, future events are inherently uncertain, and our actual future results or performance may be materially different from what we expect. Accordingly, you are strongly cautioned that reliance on any forward-looking statements is subject to significant known and unknown risks and uncertainties. All forward-looking statements contained herein are qualified by reference to the cautionary statements set forth in this section. All information provided in this press release is as of the date of this press release and are based on assumptions that we believe to be reasonable as of this date, and we do not undertake any obligation to update any forward-looking statement, except as required under applicable law. Investor Contact:+1-617-307-7503ir@crisprtx.com Media Contact:+1-617-315-4493media@crisprtx.com 1 Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380, 2095-1128.
Erasmus Medical Center Advances Cardiovascular Care with Genesis Robotic System
ST. LOUIS, Sept. 22, 2025 (GLOBE NEWSWIRE) — Stereotaxis (NYSE: STXS), a pioneer and global leader in surgical robotics for minimally invasive endovascular intervention, today announced successful first procedures by physicians at Erasmus University Medical Center in Rotterdam, The Netherlands, using the advanced Genesis Robotic Magnetic Navigation System. “We have long recognized the clinical benefits of robotics, and are delighted to be the first in The Netherlands to make the Genesis robotic system available to cardiovascular patients,” said Dr. Sing-Chien Yap, Director of Electrophysiology at Erasmus Medical Center. “We are impressed with the speed and responsiveness of Genesis. Combined with the MAGiC Catheter we are particularly pleased with the synergistic additive improvements to robotic magnetic navigation. These technological advances are important in ensuring we can offer the best care to all arrhythmia patients, including children, patients with congenital heart disease, and patients with complex arrhythmia.” Erasmus MC has been a global leader in using robotic magnetic navigation for complex arrhythmia procedures, having performed over 4,500 procedures with Stereotaxis technology. The Genesis System is the latest advancement in Robotic Magnetic Navigation technology. Robotic Magnetic Navigation introduces the benefits of robotic precision and safety to cardiac ablation, a common minimally invasive procedure to treat arrhythmias. Tens of millions of individuals worldwide suffer from arrhythmias – abnormal heart rhythms that result when the heart beats too quickly, too slowly, or with an irregular pattern. When left untreated, arrhythmia may significantly increase the risk of stroke, heart failure, and sudden cardiac arrest. “We appreciate our long-term partnership with the electrophysiology team at Erasmus,” said David Fischel, Stereotaxis Chairman and CEO. “We look forward to continuing to support their successful robotic program and working together to advance technological progress, scientific discovery, and clinical care.” About StereotaxisStereotaxis (NYSE: STXS) is a pioneer and global leader in innovative surgical robotics for minimally invasive endovascular intervention. Its mission is the discovery, development and delivery of robotic systems, instruments, and information solutions for the interventional laboratory. These innovations help physicians provide unsurpassed patient care with robotic precision and safety, expand access to minimally invasive therapy, and enhance the productivity, connectivity, and intelligence in the operating room. Stereotaxis technology has been used to treat over 150,000 patients across the United States, Europe, Asia, and elsewhere. For more information, please visit www.stereotaxis.com This press release includes statements that may constitute “forward-looking” statements, usually containing the words “believe”, “estimate”, “project”, “expect” or similar expressions. Forward-looking statements inherently involve risks and uncertainties that could cause actual results to differ materially. Factors that would cause or contribute to such differences include, but are not limited to, the Company’s ability to manage expenses at sustainable levels, acceptance of the Company’s products in the marketplace, the effect of global economic conditions on the ability and willingness of customers to purchase its technology, competitive factors, changes resulting from healthcare policy, dependence upon third-party vendors, timing of regulatory approvals, the impact of pandemics or other disasters, statements relating to our recent acquisition of APT, including any benefits expected from the acquisition, and other risks discussed in the Company’s periodic and other filings with the Securities and Exchange Commission. By making these forward-looking statements, the Company undertakes no obligation to update these statements for revisions or changes after the date of this release. There can be no assurance that the Company will recognize revenue related to its purchase orders and other commitments because some of these purchase orders and other commitments are subject to contingencies that are outside of the Company’s control and may be revised, modified, delayed, or canceled. Stereotaxis Contacts: David L. FischelChairman and Chief Executive Officer Kimberly PeeryChief Financial Officer 314-678-6100Investors@Stereotaxis.com
Genesis MedTech J-VALVE® TF Approved by NMPA, China’s First Transfemoral TAVR for Aortic Regurgitation
Clinical data demonstrate low mortality, fewer complications and faster patient recovery with a minimally invasive alternative to open-heart surgery SINGAPORE, Sept. 17, 2025 /PRNewswire/ — Genesis MedTech’s structural heart subsidiary, Suzhou Jiecheng, has received approval from China’s National Medical Products Administration (NMPA) for the J-VALVE® Transfemoral Transcatheter Aortic Valve Replacement (TAVR) System (J-VALVE TF), the […]
Jupiter Endovascular wins 510(k) FDA clearance for Vertex catheter
Jupiter Endovascular’s Vertex™ Catheter just received 510(k) clearance for the insertion of endovascular devices. This technology adopts Transforming Fixation (TFX) — able to shift from flexible to firm on demand, giving physicians true stability and control. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K252027
Argá Medtech Announces First Enrollments in COHERENT-AF IDE Clinical Trial
– Second generation pulsed field ablation system will be evaluated in paroxysmal and persistent atrial fibrillation patients to gain FDA approval – LAUSANNE, Switzerland and SAN DIEGO, Sept. 18, 2025 /PRNewswire/ — Argá Medtech, developers of the Coherent Sine-Burst Electroporation™…