GUILFORD, Conn., June 29, 2022 (GLOBE NEWSWIRE) — Hyperfine, Inc. (Nasdaq: HYPR), the groundbreaking medical device company that created Swoop®, the world’s first FDA-cleared portable MRI system™, today announced that Dave Scott will step down from his role as President, Chief Executive Officer (CEO), and Board Member for personal reasons, effective July 29, 2022. Scott Huennekens, […]
Other News
Reflow Medical Completes DEEPER LIMUS Clinical Trial Enrollment
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–Reflow Medical, Inc., a California-based medical device company, has completed enrollment in the DEEPER LIMUS clinical trial (NCT04162418) to evaluate the Temporary Spur Stent System, a patented device designed to treat long, diffuse and severely calcified infrapopliteal disease. The system allows for uniform expansion of the stent […]
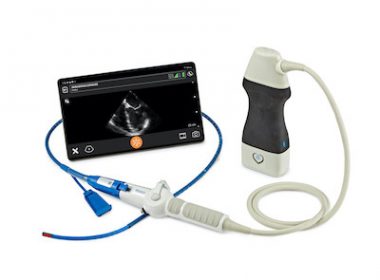
Clarius Introduces Partnership with ImaCor That Powers New FDA Cleared Handheld Hemodynamic Ultrasound
Now made ultra-portable with Clarius, ImaCor’s Zura Handheld Hemodynamic Ultrasound™ has received FDA clearance as the world’s first handheld transesophageal echocardiography (TEE) system, enabling more clinicians to accurately determine appropriate interventions in real-time. VANCOUVER, BC and JERICHO, N.Y., June 29, 2022 /PRNewswire/ — Clarius Mobile Health, a leading provider of high-definition wireless ultrasound systems, and ImaCor Inc, […]
Humacyte Announces JAMA Surgery Publication Highlighting Potential of Human Acellular Vessel™ (HAV™) to Expand Vascular Trauma Reconstruction and Bypass Treatment Options
— HAV implanted in nearly 500 patients with more than 1,000 patient-years of follow up to date, for treatment of peripheral arterial disease, arteriovenous access for hemodialysis, and trauma – — Immediately-available HAV, if approved, would represent significant and innovative advancements for vascular repair and replacement conduits– DURHAM, N.C., June 29, […]
Nuwellis Announces First Patient Enrolled in its Pivotal Trial REVERSE-HF
REVERSE-HF will evaluate ultrafiltration therapy in comparison to IV diuretics to treat heart failure patients with fluid overload MINNEAPOLIS, June 29, 2022 (GLOBE NEWSWIRE) — Nuwellis, Inc. (Nasdaq: NUWE) today announced the first patient has been enrolled in the company’s pivotal REVERSE-HF (Ultrafiltration Versus IV Diuretics in Worsening Heart Failure) clinical study. REVERSE-HF will evaluate […]
Windtree Is Leveraging Positive Istaroxime Early Cardiogenic Shock Results to Proactively Engage in Licensing Discussions and Explore Strategic Opportunities to Realize Greater Shareholder Value
Positive Data for Istaroxime in Early Cardiogenic Shock Brings Notable Interest from Potential Partners WARRINGTON, Pa., June 29, 2022 (GLOBE NEWSWIRE) — Windtree Therapeutics, Inc. (NasdaqCM: WINT), a biotechnology company focused on advancing late-stage interventions for acute cardiovascular disorders, today provided an update to its strategy and pursuit related to […]
Acticor Biotech Announces the Issuance of a Patent in Europe for Glenzocimab, Its Innovative Product for the Treatment of Cardiovascular Emergencies
This patent protects the use of glenzocimab in thrombotic diseases until 2036 This patent has already been granted in the United States and Singapore and is in the process of being granted in other countries such as Japan PARIS–(BUSINESS WIRE)–Regulatory News: ACTICOR BIOTECH (ISIN: FR0014005OJ5 – ALACT), a clinical-stage biotechnology […]
JenaValve Announces Results from First Commercial Trilogy Heart Valve System Implants in Europe
IRVINE, Calif., June 28, 2022 (GLOBE NEWSWIRE) — JenaValve Technology, Inc. (“JenaValve” or the “Company”), developer and manufacturer of differentiated transcatheter aortic valve replacement (TAVR) systems, announced positive results from the first Trilogy Heart Valve System commercial implants for high-surgical risk patients with severe, symptomatic aortic stenosis (AS) or aortic […]
CardieX Announces CONNEQT Pulse, the World’s First Customizable Dual Blood Pressure and Arterial Health Monitor
Announcement of 510(k) premarket submission with the FDA for CONNEQT Pulse IRVINE, Calif., June 28, 2022 /PRNewswire/ — CardieX Limited (ASX: CDX), a global health technology company focused on cardiovascular disease, has together with its manufacturing partner Andon filed a 510(k) premarket submission with the U.S. Food and Drug Administration (FDA) for the CONNEQT Pulse […]
Atlas Healthcare Partners and MedAxiom Announce First-of-its-Kind Cardiovascular ASC Solution
Partnership will transform ASC cardiovascular care delivery and expand patient access PHOENIX and NEPTUNE BEACH, Fla., June 28, 2022 /PRNewswire/ — Atlas Healthcare Partners – which specializes in developing and managing ambulatory surgery centers (ASCs) – and MedAxiom – which is dedicated to cardiovascular organizational performance improvement – today announced the formation of a first-of-its-kind cardiovascular-focused ASC company. […]