MINNEAPOLIS, May 24, 2022 /PRNewswire/ — VentureMed Group, Inc., a privately held medical device innovator in access management for arteriovenous (AV) fistulas and grafts and vessel preparation for interventional treatment of peripheral arterial disease (PAD) announced today that it has completed enrollment of a randomized clinical trial (RCT) titled “FLEX Vessel Prep Prior To […]
Other News
Cardio Diagnostics Announces Headquarters Relocation to Chicago
Pioneer of AI-driven, epigenetics-based clinical tests for cardiovascular disease moves for Chicago’s thriving biotech ecosystem and access to talent and resources CHICAGO, May 25, 2022 /PRNewswire/ — Cardio Diagnostics, Inc. (“Cardio Diagnostics”), a company pioneering AI-driven, epigenetics-based clinical tests for cardiovascular disease (CVD), today announced they have relocated their headquarters to Chicago, Illinois. Cardio Diagnostics will be […]
Dr. Thomas E. MacGillivray Named Chair of Cardiac Surgery for MedStar Heart & Vascular Institute
WASHINGTON, May 25, 2022 /PRNewswire/ — MedStar Heart & Vascular Institute is pleased to announce that Thomas E. MacGillivray, MD, has been named physician executive director of Cardiac Surgery at MedStar Health, and chairman of Cardiac Surgery at MedStar Washington Hospital Center, effective September 1, 2022. Dr. MacGillivray will join MedStar Heart & Vascular […]
ŌNŌCOR Receives FDA Clearance for Novel Endovascular Retrieval Technology
The ŌNŌ is intended to simplify bailout procedures in the expanding world of structural heart disease interventions PHILADELPHIA, May 25, 2022 (GLOBE NEWSWIRE) — ŌNŌCOR LLC, a leader in endovascular safety technology, today announced it has received 510(k) U.S. Food and Drug Administration (FDA) clearance for its ŌNŌ retrieval system, […]
Pharmazz Inc. Announces Positive Results of Phase III Clinical Trial Evaluating Sovateltide as a Treatment for Acute Cerebral Ischemic Stroke
Sovateltide produced a statistically significant and clinically meaningful improvement in neurological outcomes in acute cerebral ischemic stroke patients 90 days post-treatment Sovateltide demonstrated a highly favorable ordinal shift in the modified Rankin Scale (mRS) score and a significantly (p=0.0045) greater number of patients with an improvement of ≥2 points on the mRS […]
BioSig’s PURE EP™ System to be Featured During EPLive 2022
The Company’s flagship technology to be featured during a two-day educational conference that draws the world’s top cardiac electrophysiology experts Westport, CT, May 25, 2022 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (NASDAQ: BSGM) (“BioSig” or the “Company”), a medical technology company advancing electrophysiology workflow by delivering greater intracardiac signal fidelity […]
Imperative Care Announces 10,000 Patients Treated With Its Innovative Stroke Technologies
Company Reaches Milestone During Stroke Awareness Month, Serving as a Reminder to Learn the Signs of Stroke CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced that 10,000 patients have been treated in the U.S. with its technologies for stroke since 2020. The company’s current portfolio includes the Zoom Stroke Solution™ and […]
FDA Grants Breakthrough Device Designation to Anumana’s ECG Pulmonary Hypertension Early Detection AI Algorithm
Developed in collaboration with Janssen and Mayo Clinic, this technology has the potential to aid physicians in earlier detection of pulmonary hypertension, a progressive, life-threatening disease CAMBRIDGE, Mass.–(BUSINESS WIRE)–Anumana, Inc., an AI-driven health technology company from nference, Inc., today announced that the U.S. Food & Drug Administration (FDA) has granted Breakthrough Device […]
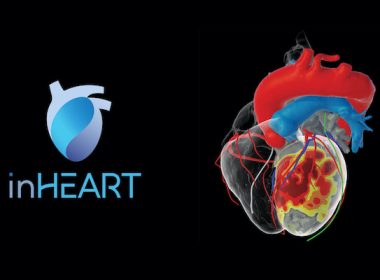
inHEART RECEIVES FDA CLEARANCE FOR NOVEL 3D CARDIAC MODELING SOLUTION
System creates 3D models of the heart with unprecedented anatomical details, allowing physicians to better plan and personalize therapeutic interventions CAMBRIDGE, Mass. and BORDEAUX, France, May 24, 2022 /PRNewswire/ — inHEART, a privately-held medical device company delivering the world’s most sophisticated digital twin of the heart, announced today that it has received FDA 510(k) clearance […]
Upstream Peripheral Technologies Partners with Red One Medical to Facilitate Access of the GoBack Catheter to the VA Health System
TAMPA, Fla.–(BUSINESS WIRE)–Upstream Peripheral Technologies announced today that it has partnered with Red One Medical for the introduction of the GoBack® Crossing and Reentry Catheter to the VA Health System. The partnership will allow physicians in the VA Health System to have access to this new technology in treating patients […]