Proposal No. 1 (The Acquisition Proposal) to be adjourned pending final NASDAQ approval; Company operations, financings and closing of the merger transaction remain not impactedLOS ANGELES and VANCOUVER, British Columbia, Sept. 02, 2025 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (“BioSig” or the “Company”), which recently merged with Streamex Exchange Corporation (“Streamex”) (NASDAQ: BSGM), today provided an update regarding the status of the merger and related proxy materials. The merger transaction closed on May 28, 2025, under the previously executed Share Purchase Agreement. As part of this transaction, 19.99% of BioSig shares are already issuable to Streamex shareholders, with the remaining issuances pending Nasdaq approval. In connection with the Company’s Schedule 14A filings, the Board of Directors has determined that, due to pending Nasdaq approval, Proposal No. 1 (the Acquisition Proposal) will not be acted upon at the September 5, 2025, Special Meeting and will instead be adjourned to a later date to be announced by the Company at the Special Meeting. While the merger with Streamex has already been consummated, the issuance of the remaining shares requires formal stockholder approval, which will be obtained at the reconvened Special Meeting. Importantly, the removal of Proposal No. 1 from the proxy does not impact: The closing of the merger;The timing of closings of the Company’s financings; orThe operations of the Company. All other proposals outlined in the Company’s definitive proxy statement will proceed as planned at the September 5, 2025 Special Meeting. About Streamex Streamex is an RWA tokenization company building Institutional grade infrastructure to bring the gold and commodities market on chain, enabled by a gold denominated treasury and tokenization technology powering the modern commodities market. Streamex is a wholly owned subsidiary of BioSig Technologies, Inc. About BioSig Technologies BioSig Technologies, Inc. is a medical device technology company with an advanced digital signal processing technology platform, the PURE EP™ Platform that delivers insights to electrophysiologists for ablation treatments of cardiovascular arrhythmias. Forward-Looking Statements This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential,” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions, and are subject to various known and unknown risks and uncertainties, many of which are beyond our control. It is possible that our actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated in these forward-looking statements, depending on factors including whether we will be able to realize the benefits of the acquisition of Streamex, whether shareholder approval of the acquisition will be obtained, and whether we will be able to maintain compliance with Nasdaq’s listing criteria in connection with the acquisition and otherwise. For a discussion of other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in forward-looking statements, see our filings with the Securities and Exchange Commission, including the section titled “Risk Factors” in our Annual Report on Form 10-K, filed with the SEC on April 15, 2025. We assume no obligation to publicly update or revise our forward-looking statements as a result of new information, future events or otherwise, except as required by law. Contacts BioSig/Streamex Press & Investor Relations: Adele CareyAlliance Advisors Investor Relationsacarey@allianceadvisors.comHenry McPhieCEO of BioSig, Co-Founder of Streamexcontact@Streamex.comhttps://www.streamex.com/https://x.com/streamex
Other News
Compelling data at ESC Congress 2025 reinforce safety and performance of the Medtronic OmniaSecure™ defibrillation lead, including in the left bundle branch area
The upcoming U.S. launch of the small-diameter OmniaSecure defibrillation lead will introduce the first FDA-approved lead for both adults and adolescent pediatric patients Cardiac Rhythm Management At the 2025 European Society of Cardiology (ESC) Congress in Madrid, researchers presented three new datasets reinforcing the safety and performance of the OmniaSecure™ […]
Medtronic receives approval for Symplicity Spyral™ Renal Denervation System in Japan
Japan is now the 77th country to receive regulatory approval for the Symplicity Spyral Renal Denervation System Medtronic, a global leader in healthcare technology, today announced it received approval in Japan from the Pharmaceuticals and Medical Devices Agency (PMDA) for its Symplicity Spyral™ renal denervation (RDN) system, also known as […]
SCD-PROTECT publication: High SCD risk despite modern GDMT across 19,500+ LifeVest® patients
September 2, 2025 – CHELMSFORD, MASS. – ZOLL®, an Asahi Kasei company that manufactures medical devices and related software solutions, is highlighting the recent European Heart Journal publication of SCD-PROTECT, an independent clinical trial designed to specifically evaluate the risk of sudden cardiac death (SCD) in the early post-MI/CAD and […]
Medtronic Evolut TAVR systems receive FDA approval for expanded Redo TAVR indication
Medtronic plc, a global leader in healthcare technology, today announced it received U.S. Food and Drug Administration (FDA) approval for the expanded Redo-TAVR indication of the Evolut™ transcatheter aortic valve replacement (TAVR) system. This approval allows for the implantation of a new Evolut transcatheter aortic valve (TAV) inside any failed […]
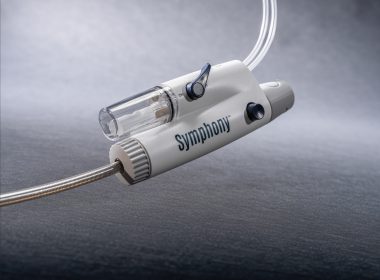
Imperative Care Announces FDA Clearance of Symphony Thrombectomy System, the First Large-Bore Continuous Vacuum System for Treatment of Pulmonary Embolism
CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its Symphony® Thrombectomy System to treat pulmonary embolism (PE), a life-threatening condition caused by blood clots blocking an artery in the lungs. This clearance expands the use of Symphony – previously for the treatment of […]
E2 (Endovascular Engineering, Inc.) Appoints Justin Farry as Chief Financial Officer to Support Next Phase of Growth
MENLO PARK, Calif., Sept. 2, 2025 /PRNewswire/ — E2 (Endovascular Engineering, Inc.) a medical device company advancing endovascular therapies for the treatment of venous thrombo-embolism (VTE), today announced the appointment of Justin Farry as its new Chief Financial Officer. A…
Windtree Therapeutics Stockholders Approve Key Proposals for Revenue and Profit Generation at the Special Stockholder Meeting
The Special Stockholder Meeting on August 28, 2025 recorded the proxy voting approvals of all ten of the Windtree proposals by stockholders The stockholder approved proposals provide the foundation for Windtree revenue generating deals in environmental services Windtree is choosing to focus on environmental services and other revenue generating businesses – the Company will not move forward with the cryptocurrency treasury strategy Company continues to move forward with potential deals for both biotech assets that will bring in near term cash and long term milestones and royalties WARRINGTON, Pa., Sept. 02, 2025 (GLOBE NEWSWIRE) — Windtree Therapeutics, Inc. (“Windtree” or “the Company”) (OTCID: WINT), a diversified company with several divisions and focused on becoming a revenue generating company, announced that key proposals for its corporate strategy were approved at the Special Stockholder Meeting on August 28, 2025. The proxy voting reached a quorum prior to the meeting date and all ten of the proposals were approved by stockholder proxy voters. Two proposals were related to the environmental services company transaction planned by the Company. That transaction will provide a path to revenue generation and the opportunity to add additional environmental services companies for scale. The corporate plan targets potential profitability for the Windtree Environmental Services division when a future acquisition is completed. Another key area for the Company approved by stockholders is planning for growth through potential deals by increasing authorized shares from 125 million to 1 billion. Flexibility of financial instruments that could utilize equity is provided by this stockholder approved proposal. To ensure we are focused on our core strategy and as previously stated by the Company, it plans to find partnership for the cardiovascular and oncology biotech assets for continued development. In 2024, Windtree had $8.8 million of expense for research and development (R&D) related to the cardiovascular program. The Company believes that a partnership could eliminate further R&D expenses. The Company believes that focusing on its new core business will be beneficial for its stockholders. The Company has chosen not to move forward with the cryptocurrency treasury strategy. “We thank our stockholders for supporting our strategic plan in their proxy voting,” said Jed Latkin, Chief Executive Officer of Windtree. “Our forward-looking plan to generate revenue and future profit with our assets is underway and we are making progress every day. We believe that focusing on our new core business will be beneficial to our stockholders. As such, we have chosen not to move forward with the cryptocurrency treasury strategy and we hope to successfully partner our biotech assets in the near term, which will eliminate R&D expenses which were nearly $9 million in 2024. We look forward to providing updates on this Company transformation.” About Windtree Therapeutics, Inc.Windtree Therapeutics, Inc. is a diversified company with several divisions and focused on becoming a revenue generating company with future profitability. Forward Looking StatementsThe Company may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are based on information available to the Company as of the date of this press release and are subject to numerous important factors, risks, and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. Examples of such risks and uncertainties include, among other things: risks related to the Company’s ability to begin its environmental services business, wind down the crypto currency strategy, and manage costs and execute on its operational and budget plans. These and other risks are described in the Company’s periodic reports, including its Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K, filed with or furnished to the Securities and Exchange Commission and available at www.sec.gov. Any forward-looking statements that the Company makes in this press release speak only as of the date of this press release. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events, or otherwise, after the date of this press release. Contact Information:Eric Curtisecurtis@windtreetx.com
Sotagliflozin Provides Consistent Relative Risk Reduction in Heart Failure and Major Cardiovascular Events Across All Age Ranges, Including Greater Than 75, in Data Presented at the European Society of Cardiology (ESC) 2025 Congress
Oral presentation highlighted improvements in heart failure endpoints and major adverse cardiovascular events (MACE) among older adults irrespective of ageTHE WOODLANDS, Texas, Sept. 02, 2025 (GLOBE NEWSWIRE) — Lexicon Pharmaceuticals, Inc. (Nasdaq: LXRX) announced that a post-hoc analysis (“Efficacy of Sotagliflozin Among Older Adults: A Pooled Analysis of SCORED and SOLOIST-WHF”) of clinical data was presented Sunday, August 31, during an oral presentation at the European Society of Cardiology (ESC) 2025 Congress in Madrid, Spain. It is well-established that the incidence of stroke and myocardial infarction (MI, or heart attack), known collectively as major cardiovascular adverse events (MACE), rises with age, as does incidence of heart failure (HF) events. Sotagliflozin, a dual sodium-glucose cotransport 1 and 2 (SGLT-1 and SGLT-2) inhibitor, was approved by the FDA based on its demonstrated efficacy in improving HF endpoints in patients with chronic kidney disease (CKD) or HF. This latest Lexicon-funded analysis examined how the efficacy of sotagliflozin varies with age, particularly among older adults, and used data pooled from the two previous pivotal Phase 3 studies of sotagliflozin, SCORED and SOLOIST-WHF. The robust data set included nearly 12,000 participants, about 70% of whom were 65 or older. This age group represents a very large population in which type 2 diabetes, CKD and/or worsening HF are relatively common. Patients were evaluated by age, both categorically (≥65 years vs
SeaStar Medical Announces Newly Published QUELIMMUNE Health Economic Study Projecting Significantly Reduced Health Care Costs in the Treatment of Pediatric AKI due to Estimated Shorter Hospital Stays and Increased Survival
Study shows approximately 18% savings per hospitalizationDENVER, Sept. 02, 2025 (GLOBE NEWSWIRE) — SeaStar Medical Holding Corporation (Nasdaq: ICU), a commercial-stage healthcare company focused on transforming treatments for critically ill patients facing organ failure and potential loss of life announced today the publication of a health economic analysis estimating significant cost savings with the use of the QUELIMMUNE therapy in pediatric patients with Acute Kidney Injury (AKI) in the ICU setting. The analysis, as presented in the Journal of Medical Economics (IJME) estimates a cost savings of $69,146 per hospitalization when the QUELIMMUNE therapy is administered compared to modeled hospitalization costs with standard continuous renal replacement therapy (CRRT). These projected savings are estimated to offset the cost of the QUELIMMUNE therapy with a potential for institutions to incur no out-of-pocket costs for 6 days (median duration) of treatment of pediatric AKI. “Data from the QUELIMMUNE clinical studies showed organ sparing and life-saving benefits for critically ill pediatric patients with AKI requiring CRRT,” stated Kevin Chung, MD, Chief Medical Officer of SeaStar Medical and co-author of the publication. “Our analysis shows that the QUELIMMUNE therapy may “pay for itself” with the projected savings.” Dr. Chung continued, “Many of the most highly regarded children’s medical centers have already adopted the QUELIMMUNE therapy, including, most recently, a prominent pediatric hospital in Philadelphia. We believe that this added economic benefit will support broader adoption of QUELIMMUNE therapy with a clear understanding of its beneficial implications in patient care.” Highlights from the IJME publication include: A historical matched analysis of pediatric patients receiving CRRT without QUELIMMUNE from the Prospective Pediatric CRRT (ppCRRT) Registry found significantly better survival with QUELIMMUNE versus CRRT alone (adjusted odds ratio 13.4; P=0.01). Bayesian analysis indicated a 98% probability of higher survival odds with the QUELIMMUNE therapy, with a predicted risk difference of 22.4%. There are no other approved selective therapies in the US for pediatric patients with AKI due to sepsis on CRRT. These data were also previously published in 2024 in Kidney Medicine.Given the encouraging survival rates among critically ill children treated with the QUELIMMUNE therapy, the objective of the health economic study was to determine its financial impact to a health care system from an inpatient perspective by combining publicly available hospitalization cost data with non-cost clinical metrics derived from prior QUELIMMUNE studies and estimating the effect of the QUELIMMUNE therapy on inpatient hospital costs among a pediatric population receiving CRRT.Analysis of matched patient data sets to patients in the QUELIMMUNE studies (N=22) were derived from two pediatric patient registries: the Kid’s Inpatient Database (KID) Registry (N=106) and the ppCRRT Registry (N=210).Modeled hospitalization costs from the KID and ppCRRT cohorts were $457,092 and $389,451 respectively, versus a lower estimated cost of $320,304 for pediatric patients treated with the QUELIMMUNE therapy for a median of 6 days. Compared to the ppCRRT Registry cohort, the QUELIMMUNE therapy yields an estimated savings of $69,146 per hospitalization. The cost reduction is driven by two key metrics: reduced hospital length of stay of approximately 3 days and improved survival. QUELIMMUNE was approved by the US Food and Drug Administration (FDA) in 2024 under a Humanitarian Device Exemption for pediatric patients with AKI due to sepsis or a septic condition on antibiotic therapy and requiring Renal Replacement Therapy (RRT). Clinical data from the FDA application, subsequently published in Kidney Medicine, showed a 77% survival rate in patients treated with QUELIMMUNE versus standard of care, representing a potential ~50% reduction in loss of life compared to historical data in this patient population. No dialysis was required for survivors at Day 60 after QUELIMMUNE treatment. SeaStar Medical is currently conducting the NEUTRALIZE-AKI pivotal trial to evaluate the safety and efficacy of the SCD therapy in 200 patients with AKI in the ICU receiving CRRT. It has received FDA Breakthrough Device Designation for this indication and five others, including: Systemic inflammatory response in adult cardiac surgerySystemic inflammatory response in pediatric cardiac surgery to prevent post-operative adverse complications and outcomesAdult cardiorenal syndrome awaiting left ventricular assist device (LVAD) implantationEnd-stage renal disease (ESRD) requiring chronic dialysisAdult hepatorenal syndrome (HRS) About Acute Kidney Injury (AKI) and Hyperinflammation AKI is characterized by a sudden and temporary loss of kidney function and can be caused by a variety of conditions such as sepsis, severe trauma, surgery, and COVID-19. AKI can cause destructive hyperinflammation, which is the overproduction or overactivity of inflammatory effector cells and other molecules that can be toxic. Damage resulting from this destructive hyperinflammation in AKI can progress to other organs, such as the heart or liver, and potentially to multi-organ dysfunction or even failure that could result in worse outcomes, including increased risk of death. Even after resolution, these patients may face complications including chronic kidney disease or end-stage renal disease (ESRD) requiring dialysis. Extreme hyperinflammation may also contribute to added healthcare costs, such as prolonged ICU stays and increased reliance on dialysis and mechanical ventilation. About QUELIMMUNE The QUELIMMUNE (SCD-PED) therapy is SeaStar Medical’s first commercial product based on its patented Selective Cytopheretic Device (SCD) technology. The QUELIMMUNE™ therapy is being commercialized for children (age 22 or younger) with AKI and sepsis or a septic condition weighing 10 kilograms or more who are on antibiotics and being treated in the ICU with Renal Replacement Therapy (RRT). It was approved in February 2024 under a Humanitarian Device Exemption application that requires medical institutions to also participate in the SAVE Surveillance Registry and obtain Institutional Review Board approval prior to adoption and use of the QUELIMMUNE therapy. This prolongs the adoption timeline by medical institutions, but provides important data on the use of QUELIMMUNE in the “real-world” setting. Data from two clinical studies of the QUELIMMUNE therapy, published in Kidney Medicine, showed a 77% survival rate in patient treated with QUELIMMUNE versus standard of care, representing a potential ~50% reduction in loss of life compared to historical data in this patient population. No dialysis was required for survivors and 87.5% of survivors had normal kidney function at Day 60 after ICU discharge. In April 2025, SeaStar Medical was awarded the 2025 Corporate Innovator Award by the National Kidney Foundation for its significant contribution to improving the lives of pediatric patients with AKI based on the approval and introduction of the QUELIMMUNE therapy. About NEUTRALIZE-AKI Pivotal Trial The NEUTRALIZE-AKI (NEUTRophil and monocyte deActivation via SeLective Cytopheretic Device – a randomIZEd clinical trial in Acute Kidney Injury) pivotal trial is evaluating the safety and efficacy of the SCD therapy in 200 adults with AKI in the ICU receiving CRRT. The trial’s primary endpoint is a composite of 90-day mortality or dialysis dependency of patients treated with the SCD therapy in addition to CRRT as the standard of care, compared with the control group receiving only CRRT standard of care. Secondary endpoints include mortality at 28 days, ICU-free days in the first 28 days, major adverse kidney events at Day 90 and dialysis dependency at one year. The study will also include subgroup analyses to explore the effectiveness of the SCD therapy in AKI patients with sepsis and acute respiratory distress syndrome. About the SeaStar Medical Selective Cytopheretic Device (SCD) Therapy The SCD therapy is designed as a disease-modifying device that neutralizes over-active immune cells and stops the cytokine storm that yields destructive hyperinflammation and creates a cascade of events that wreak havoc in the patient’s body. The SCD therapy is designed for broad applications in multiple acute and chronic kidney and cardiovascular diseases, representing patients who today have no FDA-approved options for treating their disease. Unlike pathogen removal and other blood-purification tools, the SCD therapy is integrated with an existing continuous renal replacement therapy (CRRT) hemofiltration system to selectively target and transition proinflammatory monocytes to a reparative state and promote activated neutrophils to be less inflammatory. This unique immunomodulation approach may promote long-term organ recovery, eliminate the need for future CRRT, including dialysis, and prevent loss of life. About SeaStar Medical SeaStar Medical is a commercial-stage healthcare company focused on transforming treatments for critically ill patients facing organ failure and potential loss of life. The QUELIMMUNE (SCD-PED) therapy is SeaStar Medical’s first commercial product based on its patented Selective Cytopheretic Device (SCD) technology. It was approved in 2024 by the U.S. Food and Drug Administration (FDA). QUELIMMUNE is the only FDA approved product for the ultra-rare condition of life-threatening acute kidney injury (AKI) due to sepsis or a septic condition in critically ill pediatric patients. SeaStar’s Selective Cytopheretic Device (SCD) therapy has been awarded Breakthrough Device Designation for six therapeutic indications by the FDA, enabling the potential for a speedier pathway to approval and preferable reimbursement dynamics at commercial launch. The company is currently conducting a pivotal trial of its SCD therapy in adult patients with AKI requiring continuous renal replacement therapy (CRRT), a life-threatening condition with no effective treatment options that impacts over 200,000 adults in the US annually. For more information visit www.seastarmedical.com or visit us on LinkedIn or X. Forward-Looking Statements This press release contains certain forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1955. These forward-looking statements include, without limitation, SeaStar Medical’s expectations with respect to anticipated patient enrollment and the expansion of the clinical trial sites; the total addressable market for adult SCD applications; the ability of SeaStar Medical to gain market share and generate sales with respect to the total addressable market for adult SCD applications; the ability of SCD to treat patients with AKI and other diseases; the expected regulatory approval process and timeline for commercialization; and the ability of SeaStar Medical to meet the expected timeline. Words such as “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions are intended to identify such forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to significant risks and uncertainties that could cause the actual results to differ materially from the expected results. Most of these factors are outside SeaStar Medical’s control and are difficult to predict. Factors that may cause actual future events to differ materially from the expected results include, but are not limited to: (i) the risk that SeaStar Medical may not be able to obtain regulatory approval of its SCD product candidates; (ii) the risk that SeaStar Medical may not be able to raise sufficient capital to fund its operations, including current or future clinical trials; (iii) the risk that SeaStar Medical and its current and future collaborators are unable to successfully develop and commercialize its products or services, or experience significant delays in doing so, including failure to achieve approval of its products by applicable federal and state regulators, (iv) the risk that SeaStar Medical may never achieve or sustain profitability; (v) the risk that SeaStar Medical may not be able to secure additional financing on acceptable terms; (vi) the risk that third-party suppliers and manufacturers are not able to fully and timely meet their obligations, (vii) the risk of product liability or regulatory lawsuits or proceedings relating to SeaStar Medical’s products and services, (viii) the risk that SeaStar Medical is unable to secure or protect its intellectual property, and (ix) other risks and uncertainties indicated from time to time in SeaStar Medical’s Annual Report on Form 10-K, including those under the “Risk Factors” section therein and in SeaStar Medical’s other filings with the SEC. The foregoing list of factors is not exhaustive. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and SeaStar Medical assumes no obligation and do not intend to update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise. Contact: IR@SEASTARMED.COM