FORT MILLS, S.C., Aug. 18, 2025 (GLOBE NEWSWIRE) — Catheter Precision, Inc. (VTAK – NYSE/American), a US based medical device company focused on developing technologically advanced products for the cardiac electrophysiology market announced registration and approval in the United Kingdom for its LockeT product, a suture retention device. LockeT received CE Mark for European approval and distribution in May 2025. Obtaining registration was the final approval required to launch sales in the UK.
Other News
OneMedNet initiates new Subscription Revenue model in partnership with Circle CVI
MINNEAPOLIS, Aug. 18, 2025 (GLOBE NEWSWIRE) — OneMedNet Corporation (Nasdaq:ONMD) (“OneMedNet” or the “Company”), a leader in AI-powered Real-World Data (RWD) announced a long-term strategic partnership with Circle Cardiovascular Imaging Inc. (Circle CVI), a global leader in advanced cardiovascular imaging solutions. The collaboration reflects Circle CVI’s commitment to leveraging trusted, high-quality data to drive innovation in cardiac diagnostics, treatment planning, and AI model development with data being delivered in the next 30 days. Circle CVI selected OneMedNet’s iRWD™ platform to access diverse and regulatory-grade imaging, ECG, and other cardiovascular datasets that align with the stringent requirements of healthcare AI innovation. By partnering with OneMedNet, Circle CVI gains secure, de-identified access to a growing network of real-world cardiovascular imaging data from over 1,750 provider sites — spanning over 131 million exams critical to developing, validating, and improving advanced cardiovascular solutions. “Our initial experience with OneMedNet was exceptional — the quality, depth, and regulatory readiness of the data exceeded our expectations. It quickly became clear that this wasn’t just a one-time transaction, but the beginning of a long-term collaboration. That’s why we made the decision to partner with OneMedNet and move forward with a subscription — so we can continually access the high-quality, Real-World imaging data we trust to power our innovation,” said Erkan Akyuz, CEO of Circle CVI. This strategic collaboration enables Circle CVI to expand its research and development capabilities while ensuring alignment with evolving regulatory and clinical evidence standards. It also exemplifies a growing industry shift toward real-world evidence as a foundation for medical innovation. “We’re thrilled to enter into this long-term, collaborative relationship with Circle CVI,” said Aaron Green, President and CEO of OneMedNet. “This partnership is a testament to the quality of our Real-World Data — trusted by leading healthcare companies to accelerate innovation, by meeting the precise customer requirements for longitudinal clinical data with the ultimate goal of improving patient outcomes.” About OneMedNet Corporation OneMedNet is revolutionizing how the world unlocks Real-World Data (RWD), harnessing the untapped potential of over 1,750 healthcare sites through its iRWD™ platform. This isn’t just data — it’s the lifeblood of innovation, from de-identified medical imaging to electronic health records, fueling breakthroughs for drugmakers, medical device pioneers, and AI visionaries. With a network spanning rare diseases, oncology, cardiology, and beyond, OneMedNet delivers precision insights that redefine patient care and power the next wave of healthcare disruption. Beyond healthcare OneMedNet’s proprietary AI anonymizes data for industries like finance, retail, and telecom, unlocking endless possibilities — rigorously testing production system upgrades, de-risking complex projects, and securely sharing sensitive data by stripping out personal information. Learn more at www.onemednet.com. About Circle Cardiovascular Imaging Circle Cardiovascular Imaging (Circle CVI) is a Canadian-based company that was founded in 2007, established with the aim of developing innovative software solutions to enhance cardiovascular and cerebrovascular imaging analysis and improve patient care. Circle CVI’s imaging platform provides best-in-class image reading and reporting for quantitative and qualitative assessment of cardiac MR, cardiac CT, vascular CT, and neuro CT. At the heart of Circle CVI’s operations is a relentless commitment to providing improved solutions for healthcare providers, ultimately driving better healthcare outcomes. This commitment fuels the company’s creativity, guides its decision making, and underpins its passion for innovation. Annually, millions of medical imaging exams — in over 1700 hospitals and in more than 90 countries — are estimated to be interpreted using cvi42. For additional information, please visit www.circlecvi.com or contact: marketing@circlecvi.com Cautionary Note Regarding Forward-Looking Statements This press release contains forward-looking statements. All statements other than statements of historical facts contained in this press release are forward-looking statements. We base these forward-looking statements on our current expectations and projections about future events, which we derive from the information presently available to us. Such forward-looking statements relate to future events or our future performance, including: our financial performance and projections; our growth in revenue and earnings; and our business prospects and opportunities. You can identify forward-looking statements by those that are not historical in nature, particularly those that use terminology such as “may,” “should,” “expects,” “anticipates,” “contemplates,” “estimates,” “believes,” “plans,” “projected,” “predicts,” “potential,” or “hopes” or the negative of these or similar terms. In evaluating these forward-looking statements, you should consider various factors, including: our ability to change the direction of OneMedNet; our ability to keep pace with new technology and changing market needs; the competitive environment of our business; risks inherent with investing in Bitcoin, including Bitcoin’s volatility; and our ability to implement our Bitcoin treasury strategy and its effects on our business. These and other factors may cause our actual results to differ materially from any forward-looking statement. Forward-looking statements are only predictions. The forward-looking events discussed in this press release and other statements made from time to time by us or our representatives, may not occur, and actual events and results may differ materially and are subject to risks, uncertainties, and assumptions about us. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this press release to conform these statements to actual results or to changes in our expectations. OneMedNet Contacts: Michael Wong, VP Marketing Email: michael.wong@onemednet.com SOURCE: ONEMEDNET CORPORATION
Centerline Biomedical Appoints Jim Dillon President and Chief Executive Officer
CLEVELAND–(BUSINESS WIRE)–Centerline Biomedical, Inc. (“Centerline”), an innovative leader in cardiovascular navigation and visualization systems, announced the appointment of Jim Dillon as President and Chief Executive Officer (CEO) and a member of its board of directors. He will assume his responsibilities on August 18, 2025. “It is an honor to join […]
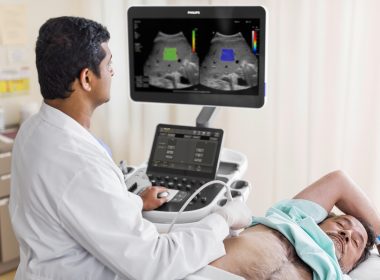
Philips Announces Plan for More Than USD 150 Million of New Investment in Manufacturing and R&D in the U.S. to Expand Production of AI-powered Health Technology Innovations
Global health technology leader, which serves 90% of U.S. hospitals, builds on top of existing U.S. investments to support local manufacturing and more than USD 900 million of annual R&D investment in the U.S. Philips EPIQ Elite Ultrasound CAMBRIDGE, Mass.–(BUSINESS WIRE)–Philips, a global leader in health technology, today announced a plan […]
Simpson Interventions Announces First Patients Treated in Clinical Trial for its Acolyte™ Catheter System
The first patients were treated at Emory University Hospital using the Acolyte Image-Guided Crossing and Re-Entry Catheter System Trial sites are planning to treat a minimum of 103 lesions, at as many as 15 sites The Acolyte Catheter System is intended to provide interventional cardiologists real-time visualization of intravascular morphology […]
Lexeo Therapeutics Reports Second Quarter 2025 Financial Results and Operational Highlights
Breakthrough Therapy designation granted for LX2006 based on interim data from Phase I/II trials demonstrating clinically meaningful improvements in cardiac and neurologic measures of Friedreich ataxia LX2006 selected for FDA Chemistry, Manufacturing, and Controls Development and Readiness Pilot (CDRP) program, created to facilitate CMC registrational readiness and support faster patient access Eight participants dosed in Phase I/II clinical trial (HEROIC-PKP2) of LX2020 for PKP2-ACM; interim clinical data update on track for second half of 2025 Strategic partnership announced with Perceptive Xontogeny Venture Funds and venBio Partners to advance non-viral, RNA-based therapeutics for genetic cardiac diseases $80 million equity financing to support development of clinical stage pipeline; cash, cash equivalents and investments in marketable securities of $152.5 million expected to provide operational runway into 2028 Louis Tamayo appointed Chief Financial Officer NEW YORK, Aug. 14, 2025 (GLOBE NEWSWIRE) — Lexeo Therapeutics, Inc. (Nasdaq: LXEO), a clinical stage genetic medicine company dedicated to pioneering novel treatments for cardiovascular diseases, today provided business updates across its portfolio and reported second quarter 2025 financial results. “Over the last several months, Lexeo has made significant progress advancing our clinical stage programs, diversifying our pipeline through a strategic partnership that we believe enables us to stay on the cusp of leading-edge cardiovascular science, and further strengthening our balance sheet,” said R. Nolan Townsend, Chief Executive Officer of Lexeo Therapeutics. “FDA Breakthrough Therapy designation for LX2006 underscores the potential of this gene therapy candidate, and we are moving as quickly as possible in close partnership with patient advocates, clinicians, and the FA community to initiate a registrational study early next year. We are also continuing to advance our LX2020 program for arrhythmogenic cardiomyopathy with eight participants dosed to date and data updates expected in the second half of this year.” Business and Program Updates LX2006 in Friedreich Ataxia (FA): Regulatory Updates: In July 2025, Lexeo received Breakthrough Therapy designation from the U.S. Food and Drug Administration (FDA) for LX2006 based on interim clinical data demonstrating clinically meaningful improvements in cardiac and neurologic measures of FA. LX2006 has also been selected to participate in the FDA Chemistry, Manufacturing, and Controls (CMC) Development Readiness Pilot (CDRP) program, created to accelerate CMC registrational readiness for therapies with expedited clinical development timelines. Lexeo expects final alignment with FDA on the LX2006 registrational study in late Q3 to early Q4 2025.Natural History: The CLARITY-FA natural history study is currently enrolling and is expected to serve as a concurrent external control arm for the planned registrational study.Safety: LX2006 continues to be generally well tolerated with no clinically significant complement activation, and no new treatment-related serious adverse events to report.Next Steps: Lexeo expects to initiate a registrational study in early 2026 with a potential efficacy readout in 2027. LX2020 in PKP2-ACM: Dosing Update: Eight participants have been dosed to date in the HEROIC-PKP2 Phase I/II clinical trial, including three participants in Cohort 1 at the low dose (2×1013 vg/kg), three participants in Cohort 2 at the high dose (6×1013 vg/kg), and two participants in dose-expansion Cohort 3 at the high dose (6×1013 vg/kg). Cohort 3 is still enrolling and up to two additional participants may be dosed in this cohort.Safety: LX2020 has been generally well tolerated with no clinically significant complement activation, and no treatment-related serious adverse events to date across all dose cohorts.Next Steps: Lexeo expects to share an interim clinical data update in the second half of 2025. Closed $80 Million Equity Financing: In May 2025, Lexeo announced the closing of an $80 million equity financing to further advance development of its clinical stage genetic medicine candidates. Lexeo anticipates that current cash, cash equivalents and marketable securities will be sufficient to fund operating and capital expenditures into 2028, through a potential efficacy readout for the registrational study of LX2006 in 2027. Research Collaboration with Perceptive Xontogeny Venture Funds and venBio Partners: In June 2025, Lexeo announced a strategic partnership to develop therapies for genetic cardiac diseases utilizing a novel non-viral RNA platform. PXV Funds and venBio will contribute up to $40 million in private equity financing to a new entity addressing cardiac genetic diseases that existing AAV platforms are unable to treat. Lexeo is contributing expertise and know-how in cardiac genetic medicines, preclinical intellectual property and technology to the partnership, with a double-digit percentage equity position in the new entity at transaction close alongside entitlement to future milestone payments, royalties, and opt-in rights to certain program(s). New Leadership Appointment: Lexeo announced today that Louis Tamayo has been appointed Chief Financial Officer. Mr. Tamayo succeeds Kyle Rasbach who remains an advisor to Lexeo. Mr. Tamayo will support Lexeo’s late-stage clinical and commercialization plans as LX2006 development accelerates and LX2020 development continues, alongside strategic planning, portfolio management, capital allocation, and other financial operations. Mr. Tamayo brings extensive commercial finance experience, having previously served as Senior Vice President at Siemens Healthineers AG, responsible for driving revenue growth and market expansion for the company’s $5 billion global diagnostics division. In this role, he built and led high-performing finance organizations that supported multiple global product launches and strategic partnerships, directed R&D capital allocation, and oversaw large-scale transformation initiatives. Prior to Siemens Healthineers, Mr. Tamayo was the Business Unit CFO for Becton, Dickinson and Company’s $1.2 billion global diabetes care business. Mr. Tamayo began his career at Pfizer where he held a series of financial, strategic, and analytical leadership roles across U.S. and international markets. Mr. Tamayo holds a BBA in Finance and Marketing from Northeastern University. Recent Data Presentations: Lexeo presented new data at the 28th American Society of Gene & Cell Therapy (ASGCT) Annual Meeting on AAV manufacturing optimization via the Company’s Sf9-baculovirus process. Data presentations reviewed the high purity and potency of Lexeo yields with improved scalability of production and reduced cost. Lexeo also presented data at the Global Cell and Gene Therapy Summit reviewing the favorable complement profile of AAVrh10 based on clinical monitoring experience across the three gene therapy studies in FA and PKP2 in which no clinically significant events related to complement activation have been observed to date. Second Quarter Financial Results Cash Position: As of June 30, 2025, cash, cash equivalents, and investments in marketable securities were $152.5 million, which Lexeo believes will be sufficient to fund operations into 2028. Research and Development Expenses: Research and Development expenses were $14.7 million for the three months ended June 30, 2025, compared to $16.6 million for the three months ended June 30, 2024. General and Administrative Expenses: General and Administrative expenses were $16.0 million for the three months ended June 30, 2025, compared to $7.0 million for the three months ended June 30, 2024. Net Loss: Net loss was $26.1 million or $0.60 per share (basic and diluted) for the three months ended June 30, 2025, compared to $21.2 million or $0.64 per share (basic and diluted) for the three months ended June 30, 2024. About Lexeo TherapeuticsLexeo Therapeutics is a New York City-based, clinical stage genetic medicine company dedicated to reshaping heart health by applying pioneering science to fundamentally change how cardiovascular diseases are treated. The Company is advancing a portfolio of therapeutic candidates that take aim at the underlying genetic causes of conditions, including LX2006 in Friedreich ataxia (FA) cardiomyopathy, LX2020 in plakophilin-2 (PKP2) arrhythmogenic cardiomyopathy, and others in devastating diseases with high unmet need. Cautionary Note Regarding Forward-Looking StatementsCertain statements in this press release may constitute “forward-looking statements” within the meaning of the federal securities laws, including, but not limited to, Lexeo’s expectations and plans regarding its current product candidates and programs and the timing for receipt and announcement of data from its clinical trials, the timing and likelihood of potential regulatory developments and approval, expectations regarding the time period over which Lexeo’s capital resources will be sufficient to fund its anticipated operations and estimates regarding Lexeo’s financial condition. Words such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “develop,” “plan” or the negative of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While Lexeo believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements. These forward-looking statements are based upon current information available to the company as well as certain estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Lexeo’s filings with the U.S. Securities and Exchange Commission (SEC)), many of which are beyond the company’s control and subject to change. Actual results could be materially different from those indicated by such forward-looking statements as a result of many factors, including but not limited to: risks and uncertainties related to global macroeconomic conditions and related volatility; expectations regarding the initiation, progress, and expected results of Lexeo’s preclinical studies, clinical trials and research and development programs; the unpredictable relationship between preclinical study results and clinical study results; delays in submission of regulatory filings or failure to receive regulatory approval; liquidity and capital resources; and other risks and uncertainties identified in Lexeo’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2025, filed with the SEC on May 12, 2025, and subsequent future filings Lexeo may make with the SEC. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Lexeo claims the protection of the Safe Harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements. Lexeo expressly disclaims any obligation to update or alter any statements whether as a result of new information, future events or otherwise, except as required by law. Media Response:Media@lexeotx.com Investor Response:Carlo Tanzi, Ph.D.ctanzi@kendallir.com Lexeo Therapeutics, Inc.Selected Financial Information(Unaudited, in thousands, except share and per share amounts) Condensed Statements of Operations Three Months Ended June 30, Six Months Ended June 30, 2025 2024 2025 2024 Operating expenses Research and development$14,721 $16,560 $31,892 $32,302 General and administrative 15,967 6,990 32,601 14,539 Total operating expenses 30,688 23,550 64,493 46,841 Operating loss (30,688) (23,550) (64,493) (46,841) Other income and expense Gain on long-term investment 3,390 – 3,390 – Other income (expense), net (14) (1) (18) (6) Interest expense (25) (35) (53) (72) Interest income 1,268 2,348 2,461 3,999 Amortization of premium on investments (34) – (46) – Total other income and expense 4,585 2,312 5,734 3,921 Loss from operations before income taxes (26,103) (21,238) (58,759) (42,920) Income taxes – – – – Net loss$(26,103) $(21,238) $(58,759) $(42,920) Net loss per common share, basic and diluted$(0.60) $(0.64) $(1.53) $(1.41) Weighted average number of shares outstanding used incomputation of net loss per common share, basic and diluted 43,573,628 33,001,946 38,372,704 30,490,892 Condensed Balance Sheet Data June 30,2025 December 31,2024 Cash, cash equivalents, and investments in marketable securities$152,506 $128,530 Total assets 176,068 146,942 Total liabilities 37,850 30,100 Total stockholders’ equity 138,218 116,842
SeaStar Medical Reports Second Quarter 2025 Financial Results and Provides Business Updates
Business highlights include: Adult NEUTRALIZE-AKI trial enrolls 31 new patients, now over 60% enrolledThree new top-rated children’s hospitals adopt QUELIMMUNE therapy for ultra-rare pediatric Acute Kidney Injury (AKI)Positive survival results reported from QUELIMMUNE SAVE Surveillance RegistryWebcast call today at 4:30 p.m. Eastern Time DENVER, Aug. 13, 2025 (GLOBE NEWSWIRE) — SeaStar Medical Holding Corporation (Nasdaq: ICU), a commercial-stage healthcare company focused on transforming treatments for critically ill patients facing organ failure and potential loss of life, announced today financial results for the three months ended June 30, 2025, and provided business updates on key initiatives. “Since the beginning of the second quarter, we have made great progress on several fronts,” said Eric Schlorff, CEO of SeaStar Medical. “We enrolled 31 additional patients in the NEUTRALIZE-AKI trial, reported three new QUELIMMUNE customers from top-rated U.S.-based children’s hospitals, announced positive survival results for the first 20 pediatric patients treated in a commercial setting with QUELIMMUNE from the SAVE Surveillance Registry, and raised additional capital to shore up our balance sheet.” Mr. Schlorff continued, “We are now focused on several important upcoming, value-creating milestones. These include the interim results for the first 100 patients in the NEUTRALIZE-AKI trial, presentation of additional data from the SAVE Surveillance Registry, and the onboarding of other notable children’s hospitals to our customer list. Also, assuming a successful outcome from our NEUTRALIZE-AKI trial, in 2026 we plan to file our Pre-Marketing Approval (PMA) application for our Selective Cytopheretic Device (SCD) therapy as an organ-sparing and life-saving treatment for adult patients with Acute Kidney Injury (AKI) requiring continuous renal replacement therapy (CRRT).” Key Business Highlights Since the beginning of the second quarter, SeaStar Medical’s key business updates include the following: Achieved the 50% patient enrollment milestone (100 patients) in the NEUTRALIZE-AKI pivotal clinical trial, triggering the interim analysis, and subsequently increased total enrollment to 125 of 200 total anticipated patients. Recommendations from the per protocol prespecified interim analysis by the trial’s independent Data Safety Monitoring Review Board (DSMB) are expected to be provided to the Company in the third quarter of 2025.Broadened the QUELIMMUNE customer base, securing three new customers from top-rated children’s hospitals.Reported positive survival data from the SAVE Surveillance Registry, evaluating the QUELIMMUNE therapy in the first 20 critically ill pediatric patients with life-threatening AKI and sepsis or a septic condition in the commercial setting. The preliminary results showed no device related safety events with the QUELIMMUNE therapy and 75% of patients survived through 28 days. These new data are on track to validate or potentially exceed a 50% reduction in loss of life compared to historical data, as reported previously in Kidney Medicine.Received agreement from the U.S. Centers for Medicare & Medicaid Services (CMS) to pay for certain expenses incurred by medical centers treating patients covered by Medicare or Medicaid who are enrolled in the NEUTRALIZE-CRS investigational clinical trial – a rare award with less than 100 clinical trials covered annually.Announced that a $2 million United States Department of Defense (DoD) grant was awarded to The Autonomous Reanimation and Evacuation (AREVA) Research Institute to support a three-year research study that will explore the application of SeaStar Medical’s SCD therapy to reduce hyperinflammation in warfighters after severe burns, inhalation injury, and infection. The grant is one of four selected out of 160 total submissions by the 2024 Military Burn Research Program and represents cutting-edge research for extracorporeal immunomodulation to reduce inflammation after severe burns, inhalation injury, and septicemia.Implemented additional cost-saving measures to increase the company’s financial runway and focus its cash resources on the development of its SCD therapy for adult patients with AKI requiring CRRT, an annual U.S. market opportunity that is 50 times larger than the U.S. pediatric AKI market.Raised $12.4 million in gross proceeds, before deducting offering-related expenses, through a public offering in June 2025 and two registered direct offerings in July and August 2025. These offerings were priced at-the-market and proceeds will support the company’s ongoing operations into 2026. Financial Results for the Second Quarter 2025 Net revenue for the three months ended June 30, 2025, was approximately $0.3 million, reflecting sales of the QUELIMMUNE pediatric SCD therapy that was approved under a Humanitarian Device Exemption in February 2024 and launched as a commercial product by SeaStar Medical in July 2024. Research and development expenses for the three months ended June 30, 2025, and 2024, were $1.0 million and $2.3 million, respectively. The decrease in research and development expenses was primarily driven by a decline in preclinical and clinical trial expenses, consulting expenses, and personnel costs. General and administrative expenses for the three months ended June 30, 2025, and 2024, were approximately $1.0 million and $2.3 million, respectively. The decrease in general and administrative expenses was the result of a decline in audit and accounting related fees, Directors’ compensation, certain Securities and Exchange Commission (SEC) fees, personnel costs, and consultant expenditures. Other expenses (net) increased approximately $1.7 million for the three months ended June 30, 2025, compared to the three months ended June 30, 2024. The increase was primarily related the decline in favorable gains from the change in fair value of liability classified warrants, a one-time financing fee, offset by the retirement of outstanding debt obligations since March 31, 2024. Net loss for the three months ended June 30, 2025, was approximately $2.0 million, or $0.18 per share on approximately 11.3 million weighted-average shares outstanding. This compares with a net loss of approximately $3.2 million, or $1.03 per share, on approximately 3.2 million weighted-average shares outstanding for the three months ended June 30, 2024. Cash at June 30, 2025 was $6.3 million, compared to $1.8 million at December 31, 2024. In July and August 2025 the Company raised an additional $8.4 million in two registered direct offerings. SeaStar Medical Second Quarter Financial Results Conference Call Date/Time:Wednesday, August 13, 2025, at 4:30 p.m. ET / 2:30 p.m. MT Webcast:The live webcast and replay can be found here. Conference ID:2078693 Dial-in numbers:1 (800) 715-9871 within the U.S. 1 (646) 307-1963 from outside the U.S. A replay of the call will be available after 7:30 p.m. ET and can be accessed as follows: The webcast replay is available here.The call replay number is 1 (609) 800-9909 and will be available through August 20, 2025. About QUELIMMUNE The QUELIMMUNE (SCD-PED) therapy is SeaStar Medical’s first commercial product based on its patented Selective Cytopheretic Device (SCD) technology. The QUELIMMUNE™ therapy is being commercialized for children (age 22 or younger) with AKI and sepsis or a septic condition weighing 10 kilograms or more who are on antibiotics and being treated in the ICU with Renal Replacement Therapy (RRT). It was approved in February 2024 under a Humanitarian Device Exemption application that requires medical institutions to also participate in the SAVE Surveillance Registry and complete Institutional Review Board approvals prior to adoption and use of the QUELIMMUNE therapy. This prolongs the adoption timeline by medical institutions, but provides important data on the use of QUELIMMUNE in the “real-world” setting. Data from two clinical studies of the QUELIMMUNE therapy, published in Kidney Medicine, showed a 77% survival rate in patient treated with QUELIMMUNE versus standard of care, representing an approximate 50% reduction in loss of life compared to historical data in this patient population. No dialysis was required for survivors and 87.5% of survivors had normal kidney function at Day 60 after ICU discharge. In January 2025, SeaStar Medical was awarded the 2025 Corporate Innovator Award by the National Kidney Foundation for its significant contribution to improving the lives of pediatric patients with AKI based on the approval and introduction of the QUELIMMUNE therapy. About NEUTRALIZE-AKI Pivotal Trial The NEUTRALIZE-AKI (NEUTRophil and monocyte deActivation via SeLective Cytopheretic Device – a randomIZEd clinical trial in Acute Kidney Injury) pivotal trial is evaluating the safety and efficacy of the SCD therapy in 200 adults with AKI in the ICU receiving CRRT. The trial’s primary endpoint is a composite of 90-day mortality or dialysis dependency of patients treated with the SCD therapy in addition to CRRT as the standard of care, compared with the control group receiving only CRRT standard of care. Secondary endpoints include mortality at 28 days, ICU-free days in the first 28 days, major adverse kidney events at Day 90 and dialysis dependency at one year. The study will also include subgroup analyses to explore the effectiveness of the SCD therapy in AKI patients with sepsis and acute respiratory distress syndrome. About Acute Kidney Injury (AKI) and Hyperinflammation AKI is characterized by a sudden and temporary loss of kidney function and can be caused by a variety of conditions such as sepsis, severe trauma, surgery and COVID-19. AKI can cause destructive hyperinflammation, which is the overproduction or overactivity of inflammatory effector cells and other molecules that can be toxic. Damage resulting from this destructive hyperinflammation in AKI can progress to other organs, such as the heart or liver, and potentially to multi-organ dysfunction or even failure that could result in worse outcomes, including increased risk of death. Even after resolution, these patients may face complications including chronic kidney disease or end-stage renal disease (ESRD) requiring dialysis. Extreme hyperinflammation may also contribute to added healthcare costs, such as prolonged ICU stays and increased reliance on dialysis and mechanical ventilation. About the SeaStar Medical Selective Cytopheretic Device (SCD) Therapy The SCD therapy is designed as a disease-modifying device that neutralizes over-active immune cells and stops the cytokine storm that yields destructive hyperinflammation and creates a cascade of events that wreak havoc in the patient’s body. The SCD therapy is designed for broad applications in multiple acute and chronic kidney and cardiovascular diseases, representing patients who today have no FDA-approved options for treating their disease. Unlike pathogen removal and other blood-purification tools, the SCD therapy is integrated with an existing continuous renal replacement therapy (CRRT) hemofiltration system to selectively target and transition proinflammatory monocytes to a reparative state and promote activated neutrophils to be less inflammatory. This unique immunomodulation approach may promote long-term organ recovery, eliminate the need for future CRRT, including dialysis, and prevent loss of life. About SeaStar Medical SeaStar Medical is a commercial-stage healthcare company focused on transforming treatments for critically ill patients facing organ failure and potential loss of life. The QUELIMMUNE (SCD-PED) therapy is SeaStar Medical’s first commercial product based on its patented Selective Cytopheretic Device (SCD) technology. The QUELIMMUNE (SCD-PED) therapy was approved in 2024 by the U.S. Food and Drug Administration (FDA). It is the only FDA approved product for the ultra-rare condition of life-threatening acute kidney injury (AKI) due to sepsis or a septic condition in critically ill pediatric patients. SeaStar’s Selective Cytopheretic Device (SCD) therapy has been awarded Breakthrough Device Designation for six therapeutic indications by the FDA, enabling the potential for a speedier pathway to approval and preferable reimbursement dynamics at commercial launch. The company is currently conducting a pivotal trial of its SCD therapy in adult patients with AKI requiring continuous renal replacement therapy (CRRT), a life-threatening condition with no effective treatment options that impacts over 200,000 adults in the U.S. annually. For more information visit www.seastarmedical.com or visit us on LinkedIn or X. Forward-Looking Statements This press release contains certain forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1955. These forward-looking statements include, without limitation, SeaStar Medical’s expectations with respect to anticipated patient enrollment and the expansion of the clinical trial sites; the total addressable market for adult SCD applications; the ability of SeaStar Medical to gain market share and generate sales with respect to the total addressable market for adult SCD applications; the ability of SCD to treat patients with AKI and other diseases; the expected regulatory approval process and timeline for commercialization; and the ability of SeaStar Medical to meet the expected timeline. Words such as “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions are intended to identify such forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to significant risks and uncertainties that could cause the actual results to differ materially from the expected results. Most of these factors are outside SeaStar Medical’s control and are difficult to predict. Factors that may cause actual future events to differ materially from the expected results include, but are not limited to: (i) the risk that SeaStar Medical may not be able to obtain regulatory approval of its SCD product candidates; (ii) the risk that SeaStar Medical may not be able to raise sufficient capital to fund its operations, including current or future clinical trials; (iii) the risk that SeaStar Medical and its current and future collaborators are unable to successfully develop and commercialize its products or services, or experience significant delays in doing so, including failure to achieve approval of its products by applicable federal and state regulators, (iv) the risk that SeaStar Medical may never achieve or sustain profitability; (v) the risk that SeaStar Medical may not be able to secure additional financing on acceptable terms; (vi) the risk that third-party suppliers and manufacturers are not able to fully and timely meet their obligations, (vii) the risk of product liability or regulatory lawsuits or proceedings relating to SeaStar Medical’s products and services, (viii) the risk that SeaStar Medical is unable to secure or protect its intellectual property, and (ix) other risks and uncertainties indicated from time to time in SeaStar Medical’s Annual Report on Form 10-K, including those under the “Risk Factors” section therein and in SeaStar Medical’s other filings with the SEC. The foregoing list of factors is not exhaustive. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and SeaStar Medical assumes no obligation and do not intend to update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise. Contact: IR@SEASTARMED.COM — Financial Tables to Follow — SeaStar Medical Holding CorporationCondensed Consolidated Balance Sheets(in thousands, except for share and per-share amounts) June 30,2025 December 31,2024 (unaudited) ASSETS Current assets Cash $6,302 $1,819 Accounts receivable, net of allowance for credit losses of $8 and $0, respectively 217 112 Inventory 77 — Prepaid expenses 1,051 1,835 Total current assets 7,647 3,766 Other assets 736 892 Total assets $8,383 $4,658 LIABILITIES AND STOCKHOLDERS’ EQUITY/(DEFICIT) Current liabilities Accounts payable $3,158 $3,046 Accrued expenses 1,883 3,188 Notes payable, net of deferred financing costs — 574 Liability classified warrants 1 33 Total current liabilities 5,042 6,841 Total liabilities 5,042 6,841 Commitments and contingencies (Note 10) Stockholders’ equity/(deficit) Preferred stock – $0.0001 par value, 10,000,000 shares authorized at June 30, 2025 and December 31, 2024; no shares issued and outstanding at June 30, 2025 and December 31, 2024 — — Common stock – $0.0001 par value per share; 450,000,000 and 500,000,000 shares authorized at June 30, 2025 and December 31, 2024, respectively; 17,343,269 and 5,977,246 shares issued and outstanding at June 30, 2025 and December 31, 2024, respectively 2 2 Additional paid-in capital 148,677 137,379 Accumulated deficit (145,338) (139,564)Total stockholders’ equity/(deficit) 3,341 (2,183)Total liabilities and stockholders’ equity/(deficit) $8,383 $4,658 SeaStar Medical Holding CorporationCondensed Consolidated Statements of Operations(unaudited)(in thousands, except for share and per-share amounts) Three Months Ended June 30, Six Months Ended June 30, 2025 2024 2025 2024 Net revenue $338 $— $631 $— Cost of goods sold 27 — 27 — Gross profit 311 — 604 — Operating expenses Research and development 1,037 2,334 3,468 4,031 General and administrative 1,030 2,335 2,716 4,588 Total operating expenses 2,067 4,669 6,184 8,619 Loss from operations (1,756) (4,669) (5,580) (8,619)Other income (expense) Interest income 45 25 93 25 Interest expense (9) (82) (18) (225)Other financing costs (298) — (298) — Change in fair value of convertible notes — (387) — (6,145)Change in fair value of warrants liability 16 1,880 32 (966)Total other income (expense), net (246) 1,436 (191) (7,311)Loss before provision for income taxes (2,002) (3,233) (5,771) (15,930)Provision for income taxes — 3 3 3 Net loss $(2,002) $(3,236) $(5,774) $(15,933)Net loss per share of common stock, basic and diluted $(0.18) $(1.03) $(0.58) $(5.36)Weighted-average shares outstanding, basic and diluted 11,329,517 3,154,782 9,981,215 2,975,248 SeaStar Medical Holding CorporationCondensed Consolidated Statements of Cash Flows(unaudited) Six Months Ended June 30, 2025 2024 Cash flows from operating activities Net loss $(5,774) $(15,933)Adjustments to reconcile net loss to net cash used in operating activities Amortization of deferred financing costs 18 57 Change in fair value of convertible notes (issued, converted and outstanding) — 6,145 Change in fair value of liability classified warrants (exercised and outstanding) (32) 966 Shares issued for the standby equity purchase agreement commitment fee 298 — Stock-based compensation 264 475 Change in operating assets and liabilities Accounts receivables, net (105) — Inventory (77) — Prepaid expenses 784 897 Other assets 156 152 Accounts payable 112 (255)Accrued expenses (1,305) 689 Other liabilities — 495 Net cash used in operating activities (5,661) (6,312) Cash flows from financing activities Proceeds from issuance of convertible notes — 979 Payment of convertible notes — (700)Proceeds from issuance of shares 5,154 4,492 Proceeds of pre-funded warrants 5,580 3,766 Proceeds from exercise of warrants 2 853 Payment of notes payable (592) (2,075)Net cash provided by financing activities 10,144 7,315 Net increase in cash 4,483 1,003 Cash, beginning of period 1,819 176 Cash, end of period $6,302 $1,179 Supplemental disclosure of cash flow information Cash paid for interest $— $439 Exercise of liability classified warrants $— $3,106 Shares issued from conversion of convertible notes $— $10,210 Issuance of convertible note warrants $— $586
BioSig Technologies Inc. Announces Pricing of $15 Million Public Offering
Los Angeles, CA, Aug. 13, 2025 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (“BioSig” or the “Company”), which recently merged with Streamex Exchange Corporation (“Streamex”) (NASDAQ: BSGM), today announced the pricing of its previously announced underwritten public offering of 3,852,149 shares of common stock at a public offering price of $3.90 per share. The offering is expected to close on or around August 15, 2025 subject to customary closing conditions. The gross proceeds from the offering, before deducting underwriter discounts and commissions and other estimated offering expenses are expected to be approximately $15,023,381.10. BioSig intends to use the net proceeds from the offering to purchase gold bullion in accordance with its investment policy, for working capital and for general corporate purposes. Clear Street and Needham & Company are acting as joint book-running managers of the offering. The offering is being made pursuant to a shelf registration statement on Form S-3 (File No. 333-276298) declared effective by the Securities and Exchange Commission (the “SEC”) on December 17, 2024. A final prospectus supplement relating to the offering will be filed with the Securities and Exchange Commission, together with an accompanying base prospectus. The securities may be offered only by means of a written prospectus forming a part of the effective registration statement. Copies of the final prospectus supplement relating to the offering, together with the accompanying base prospectus, may be obtained, when available from the SEC’s website at http://www.sec.gov, from Clear Street, Attention: Syndicate, 4 World Trade Center, 150 Greenwich St, Floor 46, New York, NY 10007, or by email at syndicate@clearstreet.io and Needham & Company, 250 Park Avenue, 10th Floor, New York, NY 10177, Attn: Prospectus Department, prospectus@needhamco.com or by telephone at (800) 903-3268. This press release shall not constitute an offer to sell or the solicitation of an offer to buy any of the securities described herein. BioSig will not, and has been advised by the joint book-running managers that they and their affiliates will not, sell any of these securities in any state or other jurisdiction in which such offer, solicitation, or sale would be unlawful prior to the registration or qualification under the securities laws of any such state or jurisdiction. About Streamex Streamex is a RWA and gold tokenization company building Institutional grade infrastructure to bring the gold market on chain, enabled by a gold denominated treasury and an institutional grade tokenization platform. Streamex is a wholly owned subsidiary of BioSig Technologies, Inc. About BioSig Technologies BioSig Technologies, Inc. is a medical device technology company with an advanced digital signal processing technology platform, the PURE EP™ Platform that delivers insights to electrophysiologists for ablation treatments of cardiovascular arrhythmias. The PURE EP™ Platform enables electrophysiologists to acquire raw signal data in real-time—absent of unnecessary noise or interference—to maximize procedural success and minimize unnecessary inefficiencies. As physician advocates, we believe that the ability to maintain the integrity of intracardiac signals with precision and clarity without driving up procedural costs has never been more pertinent. Forward-Looking Statements This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential,” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions, and are subject to various known and unknown risks and uncertainties, many of which are beyond our control. It is possible that our actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated in these forward-looking statements, depending on factors including whether we will be able to realize the benefits of the acquisition of Streamex, whether shareholder approval of the acquisition and recently announced convertible debenture financing and standby equity purchase agreement will be obtained, and whether we will be able to maintain compliance with Nasdaq’s listing criteria in connection with the acquisition and otherwise. For a discussion of other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in forward-looking statements, see our filings with the Securities and Exchange Commission, including the section titled “Risk Factors” in our Annual Report on Form 10-K, filed with the SEC on April 15, 2025. We assume no obligation to publicly update or revise our forward-looking statements as a result of new information, future events or otherwise, except as required by law. CONTACT: Press & Investor Relations:
Ele Kauderer
PR@PhoenixMGMTconsulting.com
+1 888-228-0122
Henry McPhie
CEO of BioSig, Co-Founder of Streamex
contact@Streamex.com
https://x.com/streamex
Orchestra BioMed Announces Publication of AVIM Therapy Clinical Data in JACC: Advances Demonstrating Potential to Improve Cardiac Function in Patients with Hypertension and Diastolic Dysfunction
Echocardiographic data analysis demonstrated favorable impact of atrioventricular interval modulation (“AVIM”) therapy on MODERATO II study patients with diastolic dysfunction, a key component in the development of heart failure with preserved ejection fraction (“HFpEF”)Hypertension is the leading cause of diastolic dysfunction and the most common comorbidity in older patients indicated for a pacemaker NEW HOPE, Pa., Aug. 14, 2025 (GLOBE NEWSWIRE) — Orchestra BioMed Holdings, Inc. (Nasdaq: OBIO, “Orchestra BioMed” or the “Company”), a biomedical innovation company accelerating high-impact technologies to patients through risk-reward sharing partnerships, today announced the publication of clinical data in JACC: Advances demonstrating that AVIM therapy significantly improved cardiac function in patients with hypertension and diastolic dysfunction (“DD”), key contributors to the development of HFpEF. The publication, titled “Effects of AtrioVentricular Interval Modulation (AVIM) Therapy in Subjects with Hypertension and Diastolic Dysfunction,” reports findings from a retrospective treatment-blinded analysis of MODERATO II patients. The analysis demonstrated that AVIM therapy significantly reduced systolic blood pressure (SBP) and improved echocardiographic markers of DD, a key contributor to HFpEF – a common comorbidity in patients with isolated systolic hypertension (ISH). These results were previously presented in a late-breaking session at the 2025 Technology and Heart Failure Therapeutics Meeting and are now published in JACC: Advances. Key findings: AVIM therapy significantly reduced both office and ambulatory SBP in patients with DD over 6 months, with office SBP reduced by 12.1±12.8 mmHg and ambulatory SBP by 8.3±9.7 mmHg (both p
Analytics For Life Completes Clinical Study of Non-Invasive Point-of-Care Test for Elevated Pulmonary Capillary Wedge Pressure (PCWP) Using Machine Learning
Study achieves primary sensitivity endpoint, advancing diagnostics for heart failure with preserved ejection fraction (HFpEF) and pulmonary hypertension (PH)Results build on data previously presented at ACC.25 in March 2025 TORONTO and BETHESDA, Md., Aug. 14, 2025 (GLOBE NEWSWIRE) — Analytics For Life, in collaboration with its U.S. commercial partner CorVista Health, today announced the successful completion of the clinical study evaluating a machine learning-based algorithm for non-invasively identifying patients with elevated pulmonary capillary wedge pressure (PCWP) – a key indicator of both heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF). The study met its primary endpoint for sensitivity, marking a significant milestone in the advancement in point-of-care diagnostics for heart failure patients. The results of the study will be submitted for peer-reviewed publication and presented at an upcoming scientific conference. Currently, PCWP is measured through an invasive right heart catheterization (iRHC) procedure, typically performed by a cardiologist, pulmonologist or intensivist. The CorVista capture device collects non-invasive signals for 3.5 minutes in patients at rest, potentially providing a novel method to aid in the assessment of patients being evaluated for pulmonary hypertension or heart failure. “Reaching our primary endpoint in sensitivity is a testament to the dedication of our team to pushing the boundaries of cardiovascular diagnostics. Our ability to deliver non-invasive, rapid assessment of PCWP could be a game-changer, making early detection of HFpEF, HFrEF and PH subtypes more accessible for patients,” said Charles R. Bridges, M.D., Sc.D., Executive Vice President and Chief Scientific Officer, Analytics for Life and CorVista Health. “With the clinical study now complete, we plan to initiate dialogue with regulatory agencies in preparation for the next phase of development.” Adrian Lam, President and Chief Executive Officer, CorVista Health, added, “This milestone demonstrates the power of our rapid, point-of-care solution and represents an important step toward expanding the clinical utility of the CorVista System. Adding PCWP and heart failure capabilities would allow our system to provide diagnostic support to more than 70% of all people with symptomatic cardiovascular disease – itself the leading cause of death worldwide. As we pursue a PCWP add-on, our goal is to bring advanced diagnostic accuracy into the primary care setting, enabling faster and more accurate treatment decisions while reducing the overall cost of care.” HFpEF accounts for over 50% of all heart failure cases and carries the highest rates of morbidity and mortality. Underdiagnosis of HFpEF remains one of the greatest unmet needs in cardiovascular medicine today, contributing an estimated $25 billion in direct costs to the U.S. healthcare system each year. Pulmonary hypertension, similarly underdiagnosed, requires accurate subtype classification to guide life-saving, guideline-directed therapy. The proposed PCWP add-on represents a major advancement in cardiology with far-reaching implications for early diagnosis, equitable care, and cost reduction. The CorVista System Add-On for elevated PCWP is currently an investigational device, limited by U.S. federal law to investigational use and not commercially available. About CorVista System® Developed by Analytics For Life and commercialized in the U.S. by CorVista Health, the CorVista System is a point-of-care digital health solution that detects multiple heart conditions in a single, non-invasive test. The system uses machine learned algorithms to quickly analyze heart signals and detect issues like blocked arteries or elevated pulmonary artery pressure—all in under 30 minutes, allowing clinicians to interpret results and guide treatment decisions in a single visit. The system received clearance from the U.S. Food and Drug Administration (FDA) for coronary artery disease in September 2023 and Pulmonary Hypertension in April 2024, with additional indications, including heart failure, in active development. In 2022, the CorVista System’s PH indication was awarded an FDA Breakthrough Device Designation—marking the first major advancement in PH diagnostics in over 40 years and setting a new standard for patient care. About Analytics For Life Analytics 4 Life stands at the forefront of digital health, dedicated to propelling next-generation technologies that revolutionize point-of-care diagnostics. Headquartered in Toronto, Canada, Analytics For Life specializes in pioneering non-invasive diagnostic solutions for cardiovascular diseases through the CorVista System®. The innovative CorVista System offers a non-invasive, point-of-care solution enabling physicians to assess symptomatic patients for cardiovascular disease without radiation, contrast agents, injections, fasting, or exercise. Spearheading its introduction in the United States is our exclusive commercial partner, CorVista Health. For more information on Analytics For Life, please visit: www.analytics4life.com About CorVista Health CorVista Health is on a mission to transform cardiovascular care with diagnostics that shorten the path from symptoms to diagnosis, empowering earlier treatment and better patient outcomes. We are dedicated to enabling more equitable care by providing access to immediately actionable, high-quality cardiovascular test results for previously underserved patient populations – with the goal of contributing to a future where everyone has timely access to life-saving cardiovascular care. For more information on CorVista Health, please visit: www.corvista.com Media Contact:media@corvista.com