15 Grantees Awarded Nearly $500,000 in Final 2020 Application Round CHELMSFORD, Mass.–(BUSINESS WIRE)–In the application round ended September 30, 2020, the ZOLL Foundation, established in 2014 to support young investigators through research grants in the fields of resuscitation and acute critical care, received 35 applications from across the United States, […]
Other News
SoundBite Medical Solutions Announces First Use of its Novel 0.014” Active Wire to Successfully Treat Calcified Below-The-Knee CTOs
MONTREAL–(BUSINESS WIRE)–SoundBite Medical Solutions Inc. (SBMS) announced today the first use of its novel Active Wire 0.014” platform in the successful treatment of patients suffering from critical limb ischemia (CLI) with heavily calcified below-the-knee (BTK) chronic total occlusions (CTO). The procedures were performed by Professor Marianne Brodmann, Head of the […]
Foldax Biopolymer Shown to Possess Ideal Properties for Heart Valve in New Published Paper
Company’s LifePolymer Material Used in First Polymer Heart Valves Ever Approved by FDA for Use in Clinical Trials SALT LAKE CITY–(BUSINESS WIRE)–Foldax®, Inc. today announced publication of a research paper in Advanced NanoBiomed Research that concluded that its LifePolymer™ biopolymer “exhibits ideal biomaterial properties for the flexible leaflets of a totally synthetic […]
Caladrius Biosciences Closes $25.0 Million Private Placement
BASKING RIDGE, N.J., Jan. 25, 2021 (GLOBE NEWSWIRE) — Caladrius Biosciences, Inc. (Nasdaq: CLBS) (“Caladrius” or the “Company”), a clinical-stage biopharmaceutical company dedicated to the development of cellular therapies designed to reverse disease, today announced that it has closed on its previously announced sale of an aggregate of 12,500,000 shares of […]
Amarin Expands Cardiovascular Risk Reduction Patent Infringement Lawsuit to Include Health Care Insurance Provider
DUBLIN, Ireland and BRIDGEWATER, N.J., Jan. 25, 2021 (GLOBE NEWSWIRE) — Amarin Corporation plc (NASDAQ:AMRN), announced today an expansion of the scope of its VASCEPA® (icosapent ethyl) cardiovascular (CV) risk reduction patent infringement lawsuit against Hikma Pharmaceuticals PLC to include a health care insurance provider in the United States, Health Net, […]
12-Month Data from Surmodics’ TRANSCEND Trial Presented at LINC 2021 Event
SurVeil™ Drug Coated Balloon (DCB) demonstrates non-inferior safety and efficacy, while using a substantially lower drug dose, vs. the IN.PACT® Admiral® DCB for treatment of femoropopliteal lesions. EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–Surmodics, Inc. (NASDAQ:SRDX), a leading provider of medical device and in vitro diagnostic technologies to the health care industry, today announced that […]
Kardium Announces $115M in New Financing for Innovative Atrial Fibrillation Treatment
Fidelity Management & Research Company LLC and T. Rowe Price funds lead private financing round VANCOUVER, British Columbia–(BUSINESS WIRE)–Kardium Inc., developer of the Globe® Mapping and Ablation System for the treatment of atrial fibrillation, has raised US $115 million in a new financing round. The round is led by Fidelity Management & Research […]
Alleviant Medical Receives Breakthrough Device Designation From FDA for Transcatheter Technology
Innovative technology offers an implant-free approach for individuals with chronic heart failure AUSTIN, Texas–(BUSINESS WIRE)–Alleviant Medical Inc., a privately-held medical device company, today announced that the US Food and Drug Administration (FDA) has granted the company a Breakthrough Device designation for its transcatheter technology. The technology offers a no-implant interatrial […]
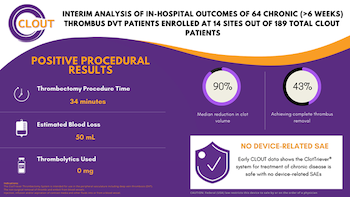
Inari Medical Announces Presentation of Positive Chronic Clot Subanalysis Results from Real World CLOUT Registry at LINC 2021
IRVINE, Calif., Jan. 25, 2021 (GLOBE NEWSWIRE) — Inari Medical, Inc. (NASDAQ: NARI) (“Inari”) a commercial-stage medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases, today announced strongly positive interim results of the first 64 chronic deep vein thrombosis (“DVT”) […]
PEDRA™ Technology Receives FDA Breakthrough Device Designation for its PEDRA™ Xauron™ Real-Time Tissue Perfusion System
Novel perfusion monitor achieves FDA Breakthrough Device Designation for real-time, periprocedural monitoring of tissue perfusion in patients with critical limb threatening ischemia SINGAPORE, Jan 25, 2021 /PRNewswire/ — PEDRA™ Technology, a privately-held company, announced today that the U.S Food and Drug Administration (FDA) has granted the company a Breakthrough Device Designation for the […]