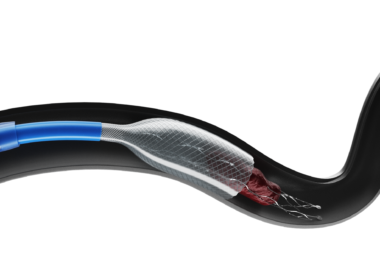
BARCELONA – June 18, 2025 – ANACONDA Biomed, a medical technology company developing next-generation thrombectomy systems for the treatment of ischemic stroke, has announced that it has received CE Mark certification for its ANA5 Funnel Catheter. The CE marking confirms that the ANA5 device complies with the European Union’s health, […]
Other News
DESKi Closes $6M Seed Round to Bring AI-Powered Heart Scans to Market
BORDEAUX, France–(BUSINESS WIRE)–DESKi, a healthtech company developing AI-powered diagnostic tools in collaboration with clinicians and researchers, today announced the close of a $6 million seed round to support the U.S. and global market launch of its FDA-approved cardiac imaging software, HeartFocus. “This funding moves us one step closer to a world […]
Crossroads Neurovascular, Inc. Announces FDA 510(k) Clearance for PATH BGC™, the World’s First 7F-Compatible Balloon Guide Catheter for Neurovascular Use
Lake Forest, CA – Crossroads Neurovascular, Inc. is proud to announce that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance (K242392) for its groundbreaking PATH BGC™, a next-generation balloon guide catheter (BGC) designed to advance neurovascular intervention. With this clearance, PATH BGC becomes the world’s first BGC […]
Orchestra BioMed Showcases AVIM Therapy as Purpose-Built Solution for Hypertensive Heart Disease at CSI Frankfurt 2025
Presentation underscores unique potential for atrioventricular interval modulation (“AVIM”) therapy to manage blood pressure in older, high-risk patients who have indicators of diastolic dysfunction and progression to heart failure with preserved ejection fraction (“HFpEF”)Hypertensive heart disease represents over 7.7 million U.S. patients, the same population with hypertension and elevated cardiovascular risk cited in the recent FDA Breakthrough Device Designation (“BDD”) for AVIM therapy NEW HOPE, Pa., June 18, 2025 (GLOBE NEWSWIRE) — Orchestra BioMed Holdings, Inc. (Nasdaq: OBIO, “Orchestra BioMed” or the “Company”), a biomedical company accelerating high-impact technologies to patients through risk-reward sharing partnerships, today announced the presentation of key clinical insights into the role of AVIM therapy for the treatment of high-risk hypertension at the Congenital, Structural, and Valvular Heart Disease Interventions (“CSI”) 2025 Meeting. The data highlight AVIM therapy’s unique potential to address hypertensive heart disease, a significant and under-recognized cardiovascular syndrome that affects a growing segment of the aging hypertension population. The talk, “Atrioventricular Interval Modulation (AVIM) Therapy for Hypertension and HFpEF,” will be delivered by Daniel Burkhoff, M.D., Ph.D., Director of Heart Failure, Hemodynamics and Mechanical Circulatory Support Research at Cardiovascular Research Foundation and clinical advisor to Orchestra BioMed. Dr. Burkhoff will spotlight the clinical utility of AVIM therapy as a novel, device-based approach to blood pressure management designed specifically for patients with hypertensive heart disease. This population has increased risk for major adverse cardiac events and currently lacks sufficient therapeutic options. The presentation will take place on June 18, 2025, at 3:33pm CEST / 9:33am ET as part of the “Interventions for Chronic Heart Failure” session. Dr. Burkhoff commented, “Hypertensive heart disease is not a singular diagnosis, but a high-risk cardiovascular syndrome driven by longstanding, uncontrolled high blood pressure which significantly increases the likelihood of adverse clinical outcomes such as stroke, myocardial infarction, diastolic dysfunction and progression to heart failure. The data I will review at CSI explore how AVIM therapy may offer a unique treatment specifically catered to this group of patients leveraging a mechanism of action designed to reduce cardiac preload and modulate autonomic nervous system responses to reduce blood pressure and improve cardiovascular function. This represents a potential paradigm shift in how we approach blood pressure management using tailored interventions designed to directly impact the complex pathophysiology of high-risk hypertension.” The presentation will cover: The clinical burden and therapeutic gaps in managing patients with high-risk hypertension and increased risk of heart failure;The growing body of clinical and mechanistic evidence demonstrating AVIM therapy’s potential to lower blood pressure and improve cardiac function; andDetails on the BACKBEAT global pivotal study, currently enrolling patients with uncontrolled hypertension who are indicated for a dual-chamber pacemaker. The study is being conducted in collaboration with Medtronic, the global leader in cardiac pacing therapy. “AVIM therapy was purpose-built to address the complex and underserved needs of patients with hypertensive heart disease, a subgroup often overlooked by conventional therapy,” said Avi Fischer, M.D., Senior Vice President of Medical Affairs and Innovation at Orchestra BioMed. “As a programmable, pacemaker-integrated solution, AVIM therapy has the potential to fit seamlessly into existing electrophysiology practices while opening the door to better outcomes in a large, underserved population. Dr. Burkhoff’s presentation at CSI Frankfurt further reinforces the growing clinical interest in AVIM therapy and highlights the significant opportunity to transform care of hypertensive heart disease, especially given our recently granted BDD status, which applies directly to this patient profile.” About Orchestra BioMed Orchestra BioMed (Nasdaq: OBIO) is a biomedical innovation company accelerating high-impact technologies to patients through risk-reward sharing partnerships with leading medical device companies. Orchestra BioMed’s partnership-enabled business model focuses on forging strategic collaborations with leading medical device companies to drive successful global commercialization of products it develops. Orchestra BioMed’s lead product candidate is AVIM therapy for the treatment of hypertension, the leading risk factor for death worldwide. Orchestra BioMed is also developing Virtue SAB for the treatment of atherosclerotic artery disease, the leading cause of mortality worldwide. Orchestra BioMed has a strategic collaboration with Medtronic, one of the largest medical device companies in the world, for development and commercialization of AVIM therapy for the treatment of hypertension in pacemaker-indicated patients, and a strategic partnership with Terumo, a global leader in medical technology, for development and commercialization of Virtue SAB for the treatment of artery disease. The Company has received four Breakthrough Device Designations from the U.S. FDA across these two core programs, reflecting the significant potential of its technologies to address high unmet needs in cardiovascular care. For further information about Orchestra BioMed, please visit www.orchestrabiomed.com, and follow us on LinkedIn. References to Websites and Social Media Platforms References to information included on, or accessible through, websites and social media platforms do not constitute incorporation by reference of the information contained at or available through such websites or social media platforms, and you should not consider such information to be part of this press release. About AVIM Therapy AVIM therapy is an investigational therapy compatible with standard dual-chamber pacemakers designed to substantially and persistently lower blood pressure. It has been evaluated in pilot studies in patients with hypertension who are also indicated for a pacemaker. MODERATO II, a double-blind, randomized pilot study, showed that patients treated with AVIM therapy experienced net reductions of 8.1 mmHg in 24-hour ambulatory systolic blood pressure (aSBP) and 12.3 mmHg in office systolic blood pressure (oSBP) at six months when compared to control patients. In addition to reducing blood pressure, clinical results using AVIM therapy demonstrate improvements in cardiac function and hemodynamics. The BACKBEAT (BradycArdia paCemaKer with atrioventricular interval modulation for Blood prEssure treAtmenT) global pivotal study will further evaluate the safety and efficacy of AVIM therapy in lowering blood pressure in patients who have systolic blood pressure above target despite anti-hypertensive medication and who are indicated for or have recently received a dual-chamber cardiac pacemaker. AVIM therapy has been granted Breakthrough Device Designation by the FDA for the treatment of uncontrolled hypertension in patients who have increased cardiovascular risk. Forward-Looking Statements Certain statements included in this press release that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements relating to the enrollment, implementation and design of the Company’s planned and ongoing pivotal trials, realizing the clinical and commercial value of the Company’s product candidates, the potential safety and efficacy of the Company’s product candidates, and the ability of the Company’s partnerships to accelerate clinical development. These statements are based on various assumptions, whether or not identified in this press release, and on the current expectations of the Company’s management and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as and must not be relied on as a guarantee, an assurance, a prediction, or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and may differ from assumptions. Many actual events and circumstances are beyond the control of the Company. These forward-looking statements are subject to a number of risks and uncertainties, including changes in domestic and foreign business, market, financial, political, and legal conditions; risks related to regulatory approval of the Company’s commercial product candidates and ongoing regulation of the Company’s product candidates, if approved; the timing of, and the Company’s ability to achieve expected regulatory and business milestones; the impact of competitive products and product candidates; and the risk factors discussed under the heading “Item 1A. Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, which was filed with the SEC on March 31, 2025 and the risk factor discussed under the heading “Item 1A. Risk Factors” in the Company’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2025, which was filed with the SEC on May 12, 2025. The Company operates in a very competitive and rapidly changing environment. New risks emerge from time to time. Given these risks and uncertainties, the Company cautions against placing undue reliance on these forward-looking statements, which only speak as of the date of this press release. The Company does not plan and undertakes no obligation to update any of the forward-looking statements made herein, except as required by law. Investor ContactSilas NewcombOrchestra BioMed Snewcomb@orchestrabiomed.com Media ContactKelsey Kirk-EllisOrchestra BioMedKkirkellis@orchestrabiomed.com
RADPAIR and Lifetrack Announce Strategic Partnership to Revolutionize Radiology Workflow and Global Access
KNOXVILLE, Tenn., June 18, 2025 /PRNewswire/ — RADPAIR, a leading innovator in AI-powered radiology automation, and Lifetrack, a next-generation medical imaging platform, have announced a strategic partnership aimed at transforming radiology workflow, reporting efficiency, and global…
Gradient Denervation Technologies Announces Acceptance into FDA’s Total Product Life Cycle Advisory Program for Development of its Pulmonary Artery Denervation System
PARIS, June 18, 2025 (GLOBE NEWSWIRE) — Gradient Denervation Technologies announced today that it has been accepted into the Total Product Life Cycle Advisory Program (TAP) Pilot from the U.S. Food and Drug Administration (FDA) for the development of its novel technology intended to treat patients with pulmonary hypertension and associated heart failure. The TAP Pilot acceptance follows the Company’s recent announcement that the FDA granted Breakthrough Device Designation for the Gradient Denervation System.
Conavi Medical Corp. (TSXV: CNVI) (OTCQB: CNVIF) Investor Webinar with Presentation and Audience Q&A
TORONTO, June 18, 2025 (GLOBE NEWSWIRE) — Conavi Medical Corp. (TSXV: CNVI) (OTCQB: CNVIF) (“Conavi Medical” or the “Company”), is pleased to invite investors and other interested parties to attend the Company’s upcoming live webinar presentation, audience Q&A and interview. CEO Tom Looby will discuss Conavi’s unique and market-leading imaging technology for minimally invasive cardiovascular procedures. The webinar will be a live, interactive online event where attendees can ask the presenters questions in real time. A recording will be available for those who cannot join the live event. Event: Radius Research Pitch, Deep Dive, and Q&A with Conavi Medical Corp. Presentation Date & Time: Tuesday, June 24th @ 4 PM ET / 1 PM PT Webcast Registration Link: https://us02web.zoom.us/webinar/register/9717496764316/WN_FVNRHuMeRAqlOSq-U3wkCA Radius Research gives individual investors access to in-depth CEO interviews with deep-dive institutional-level discussion and Q&A. Radius Research is part of Market Radius Capital, Inc. and hosted by Martin Gagel, a former top-ranked sell-side technology and special situations analyst. About Conavi Medical Corp.: Conavi Medical is focused on designing, manufacturing, and marketing imaging technologies to guide common minimally invasive cardiovascular procedures. Its patented Novasight Hybrid™ System is the first system to combine both intravascular ultrasound (IVUS) and optical coherence tomography (OCT) to enable simultaneous and co-registered imaging of coronary arteries. The Novasight Hybrid System has 510(k) clearance from the U.S. Food and Drug Administration; and regulatory approval for clinical use from Health Canada, China’s National Medical Products Administration, and Japan’s Ministry of Health, Labor and Welfare. For more information, visit conavi.com. Cautionary Statement Regarding Forward-Looking Information This news release contains “forward-looking statements” within the meaning of applicable Canadian and U.S. securities laws, which reflect the current expectations of management of Conavi’s future growth, results of operations, performance and business prospects and opportunities. Forward-looking statements are frequently, but not always, identified by words such as “may”, “would”, “could”, “will”, “anticipate”, “believe”, “plan”, “expect”, “intend”, “estimate”, “potential for” and similar expressions, although these words may not be present in all forward-looking statements. Forward-looking statements that appear in this release may include, without limitation, references to Conavi’s plans for the commercialization of its Novasight Hybrid™ System. These forward-looking statements reflect management’s current beliefs with respect to future events, and are based on information currently available to management that, while considered reasonable by management as of the date on which the statements are made, are inherently subject to significant business, economic and competitive uncertainties and contingencies which could result in actions, events, conditions, results, performance or achievements to be materially different from those projected in the forward-looking statements. Forward-looking statements involve significant risks, uncertainties and assumptions and many factors could cause Conavi’s actual results, performance or achievements to be materially different from any future results, performance or achievements that may be expressed or implied by such forward-looking statements. Such factors and assumptions include, but are not limited to, Conavi’s ability to retain key personnel; its ability to execute on its business plans and strategies; and other factors listed in the “Risk Factors” sections of the joint information circular of Conavi dated August 30, 2024 and in the final short form prospectus of Conavi dated April 15, 2025 (each of which may be viewed at www.sedarplus.com). Should one or more of these risks or uncertainties materialize, or should assumptions underlying the forward-looking statements prove incorrect, actual results, performance, or achievements may vary materially from those expressed or implied by the forward-looking statements contained in this news release. These factors should be considered carefully, and prospective investors should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in the news release are based upon what management currently believes to be reasonable assumptions and Conavi has attempted to identify important factors that could cause actual actions, events, conditions, results, performance or achievements to differ materially from those described in forward-looking statements, Conavi cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements. Except as required by law, Conavi expressly disclaims any intention or obligation to update or revise any forward-looking statements whether as a result of new information, future events or otherwise. Accordingly, investors should not place undue reliance on forward-looking statements. All the forward-looking statements are expressly qualified by the foregoing cautionary statements. Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this press release. Contacts Stefano Picone Chief Financial Officer (416) 483-0100
Conformal Medical Strengthens Intellectual Property Portfolio with Issuance of Additional Patents
The company holds 29 issued patents worldwide, covering key structural innovations in Left Atrial Appendage Occlusion (LAAO) technology, including implant design and conformability features. NASHUA, N.H., June 17, 2025 /PRNewswire/ — Conformal Medical, Inc. announced the issuance of two…
Mineralys Therapeutics Announces Positive Topline Results from Phase 2 Explore-CKD Trial of Lorundrostat for the Treatment of Hypertension in Subjects with CKD and Albuminuria
– Explore-CKD met its primary endpoint; lorundrostat 25 mg once daily achieved a 9.3 mmHg reduction in systolic blood pressure, and a 7.5 mmHg placebo-adjusted reduction (p=0.0024) at four weeks – – Lorundrostat showed a clinically meaningful reduction in the pre-defined endpoint spot urine albumin-to-creatinine ratio of 31% (p6.0 mmol/L)0/59 (0%)3/58 (5%) BP, blood pressure; UACR, Urine albumin-to-creatinine ratio; TEAE, Treatment-emergent adverse event* Primary endpoint. ** Cystatin-C formula, a surrogate biomarker of renal function not subject to MATE1 transport and elimination in the glomeruli of the kidney.*** Per protocol Systolic BP, UACR, and eGFR estimates and p values from Mixed Effects Model for a crossover trial with multiple baselines. Serious Adverse Events were reported in two subjects (3%) during the lorundrostat treatment period and none during the placebo treatment period. TEAEs leading to discontinuation occurred in one subject (2%) during the placebo treatment period and in two subjects (3%) during the lorundrostat treatment period. During lorundrostat treatment, one subject discontinued treatment due to elevation of potassium associated with reduced eGFR and one subject discontinued treatment due to reduction in eGFR alone. During the lorundrostat treatment period, an anticipated, modest decrease in mean eGFR was observed (-6.8% lorundrostat, -4.6% mL/min/1.73m2 placebo-adjusted). Reduction in eGFR is also seen with other renin-angiotensin-aldosterone pathway inhibitors, including ACE inhibitors, ARBs and mineralocorticoid receptor antagonists (MRAs). This is the result of a reduction in the deleterious over-perfusion of glomeruli due, in part, to reduced blood pressure. These findings add to a growing body of evidence supporting the efficacy and safety of aldosterone synthase inhibitors (ASIs) in addressing the underlying mechanisms of hypertension, including in individuals with comorbid CKD. The reduction in UACR observed in this trial is consistent with the potential of lorundrostat to have renal protective effects. “Prolonged elevations in blood pressure in patients with compromised renal function can damage the small blood vessels in the kidneys, further reducing their ability to function properly,” said Dr. Matthew Weir, Director of the Division of Nephrology at the University of Maryland Medical Center and Professor of Medicine at the University of Maryland School of Medicine. “The evidence generated from this trial demonstrates the unique mechanism of action and benefit of lorundrostat in lowering systolic blood pressure and UACR. Lorundrostat shows significant potential in the management of hypertension and related kidney disease.” The Explore-CKD trial was designed to provide data that augments the antihypertensive profile of lorundrostat by evaluating the efficacy and safety of lorundrostat in subjects with compromised renal function. The Company had already completed three trials of lorundrostat for the treatment of subjects with uncontrolled hypertension (uHTN), including resistant hypertension (rHTN); the pivotal Phase 3 Launch-HTN and Phase 2 Advance-HTN trials, and the Phase 2, dose-ranging, Target-HTN trial, which demonstrated clinically meaningful reductions in systolic BP and a favorable safety and tolerability profile. The Company continues to study lorundrostat in the ongoing, open-label Transform-HTN extension trial, which is evaluating long-term efficacy, safety, and tolerability. Additionally, the Explore-OSA trial, initiated in the first quarter of 2025, continues to enroll subjects with OSA and uncontrolled hypertension. Conference Call The Company’s management team will host a conference call today, June 17, 2025, at 8:00 a.m. ET. To access the call, please dial 1-877-704-4453 in the United States or 1-201-389-0920 outside the United States. A live webcast of the conference call may be found here. A replay of the call will be available on the “News & Events” page in the Investor Relations section of the Mineralys Therapeutics website (click here). About Explore-CKD The Explore-CKD trial (NCT06150924) was a randomized, double-blind, placebo-controlled, two-period, two-sequence (2×2) crossover trial. This Phase 2 trial was designed to evaluate BP reduction and safety of 25 mg QD lorundrostat when added to background treatment with an ACEi or ARB and an SGLT2 inhibitor for the treatment of hypertension in subjects with CKD subjects with an estimated glomerular filtration rate (eGFR) ≥ 30 mL/min/1.73m2 and albuminuria (UACR of 200-5,000 mg/g). The primary efficacy endpoint of the trial was change from baseline in systolic BP at week four in the active versus placebo treatment period. Exploratory endpoints included change from baseline in UACR and eGFR at week four in the active versus placebo treatment period. About CKD CKD, which is characterized by the gradual loss of kidney function, is estimated to affect more than 10% of the global population and is one of the leading causes of mortality worldwide. According to the U.S. Centers for Disease Control and Prevention (CDC), an estimated 1-in-7 (approximately 37 million) U.S. adults have CKD, and approximately 22 million people in the United States are living with both hypertension and CKD.1 The relationship between these conditions is tightly linked: sustained hypertension may contribute to impaired kidney function, and progressive decrease in kidney function may lead to worsening BP control. 2 When CKD is present in patients with hypertension, the risk of cardiovascular disease and mortality rises significantly.3 Emerging evidence points to dysregulated aldosterone as a key driver of both diseases. Excess aldosterone promotes sodium retention, vascular inflammation, and fibrosis, contributing to both uncontrolled BP and kidney injury. 4,5 Despite the availability of existing therapies, a significant proportion of patients remain uncontrolled or undertreated.6 Early detection and targeted interventions that address underlying mechanisms, such as aldosterone dysregulation, may offer the potential to slow CKD progression, reduce cardiovascular risk, and improve long-term outcomes.4 Without effective management, CKD can advance to kidney failure, requiring dialysis or transplantation.7 About Hypertension Having sustained, elevated BP (or hypertension) increases the risk of heart disease, heart attack and stroke, which are leading causes of death in the United States.8 In 2022, more than 685,000 deaths in the United States included hypertension as a primary or contributing cause. 9 Hypertension and related health issues resulted in an estimated annual economic burden of about $219 billion in the United States in 2019.10 Less than 50% of hypertension patients achieve their BP goal with currently available medications.6 Dysregulated aldosterone levels are a key factor in driving hypertension in approximately 30% of all hypertensive patients.11 About Lorundrostat Lorundrostat is a proprietary, orally administered, highly selective aldosterone synthase inhibitor being developed for the treatment of uHTN or rHTN, as well as CKD and OSA. Lorundrostat was designed to reduce aldosterone levels by inhibiting CYP11B2, the enzyme responsible for its production. Lorundrostat has 374-fold selectivity for aldosterone-synthase inhibition versus cortisol-synthase inhibition in vitro, an observed half-life of 10-12 hours and demonstrated a 40-70% reduction in plasma aldosterone concentration in hypertensive subjects. The Company has now completed four successful clinical trials of lorundrostat supporting the efficacy and safety profile while also validating aldosterone as an integral therapeutic target in uHTN and rHTN. The Company has completed two pivotal, registrational trials, including the Phase 3 Launch-HTN trial and Phase 2 Advance-HTN trial, which support the robust, durable and clinically meaningful reductions in systolic BP by lorundrostat. Lorundrostat was well tolerated in both trials with a favorable safety profile. About Mineralys Mineralys Therapeutics is a clinical-stage biopharmaceutical company focused on developing medicines to target hypertension, CKD, OSA and other diseases driven by dysregulated aldosterone. Its initial product candidate, lorundrostat, is a proprietary, orally administered, highly selective aldosterone synthase inhibitor that Mineralys Therapeutics is developing for the treatment of cardiorenal conditions affected by dysregulated aldosterone, including hypertension, CKD and OSA. Mineralys is based in Radnor, Pennsylvania, and was founded by Catalys Pacific. For more information, please visit https://mineralystx.com. Follow Mineralys on LinkedIn, Twitter and Bluesky. Forward Looking Statements Mineralys Therapeutics cautions you that statements contained in this press release regarding matters that are not historical facts are forward-looking statements. The forward-looking statements are based on our current beliefs and expectations and include, but are not limited to, statements regarding: the potential therapeutic benefits of lorundrostat; the Company’s expectation that ASIs with an SGLT2 inhibitor may provide additive clinical benefits to patients; the Company’s expectation that Advance-HTN, Launch-HTN and Explore-CKD may serve as pivotal trials in submission of a new drug application (NDA) to the U.S. Food and Drug Administration (FDA); the Company’s ability to evaluate lorundrostat as a potential treatment for CKD, OSA, uHTN or rHTN; the planned future clinical development of lorundrostat and the timing thereof; and the expected timing of commencement and enrollment of participants in clinical trials and topline results from clinical trials. Actual results may differ from those set forth in this press release due to the risks and uncertainties inherent in our business, including, without limitation: topline results that we report are based on a preliminary analysis of key efficacy and safety data, and such data may change following a more comprehensive review of the data related to the clinical trial and such topline data may not accurately reflect the complete results of a clinical trial; our future performance is dependent entirely on the success of lorundrostat; potential delays in the commencement, enrollment and completion of clinical trials and nonclinical studies; later developments with the FDA may be inconsistent with the feedback from the completed end of Phase 2 meeting, including whether the proposed pivotal program will support registration of lorundrostat which is a review issue with the FDA upon submission of an NDA; the results of our clinical trials, including the Advance-HTN and Launch-HTN trials, may not be deemed sufficient by the FDA to serve as the basis for an NDA submission or regulatory approval of lorundrostat; our dependence on third parties in connection with manufacturing, research and clinical and nonclinical testing; unexpected adverse side effects or inadequate efficacy of lorundrostat that may limit its development, regulatory approval and/or commercialization; unfavorable results from clinical trials and nonclinical studies; results of prior clinical trials and studies of lorundrostat are not necessarily predictive of future results; macroeconomic trends and uncertainty with regard to high interest rates, elevated inflation, tariffs, and the potential for a local and/or global economic recession; our ability to maintain undisrupted business operations due to any pandemic or future public health concerns; regulatory developments in the United States and foreign countries; our reliance on our exclusive license with Mitsubishi Tanabe Pharma to provide us with intellectual property rights to develop and commercialize lorundrostat; and other risks described in our filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in our annual report on Form 10-K, and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and we undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. References 1National Kidney Foundation. High Blood Pressure and Chronic Kidney Disease | National Kidney Foundation. Accessed June 2025.2Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: Core Curriculum 2019. Am J Kidney Dis. 2019;74(1):120-131.3Tonelli M, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034-2047.4Bomback AS, et al. Potential of aldosterone synthase inhibition in CKD and hypertension. Kidney Int. 2022;102(1):18-27.5Luther JM. Effects of aldosterone on the kidney and cardiovascular system. Nat Rev Nephrol. 2014;10(6):308-320.6Carey RM, et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement from the AHA. Hypertension. 2018;72(5):e53-e90.7CDC. Chronic Kidney Disease in the United States, 2021. Accessed June 2025.8CDC. Facts About Hypertension. Centers for Disease Control and Prevention. Updated September 27, 2023. Accessed June 2025. 9CDC. Underlying Cause of Death, 1999–2022 Results. CDC WONDER Online Database. Accessed June 2025.10Centers for Disease Control and Prevention. Health and Economic Benefits of High Blood Pressure Interventions. National Center for Chronic Disease Prevention and Health Promotion. Updated November 20, 2023. Accessed June 2025. 11Brown JM, et al. Primary Aldosteronism and the Pathogenesis of Hypertension. Physiol Rev. 2018;98(1):103-137. Contact:Investor Relationsinvestorrelations@mineralystx.com Media RelationsLindsay RoccoElixir Health Public RelationsEmail: lrocco@elixirhealthpr.com
Lilly to acquire Verve Therapeutics to advance one-time treatments for people with high cardiovascular risk
Verve’s leading programs aim to deliver lifelong cardiovascular risk reduction with a single dose by targeting genes strongly linked to cardiovascular disease Lilly’s established capabilities in cardiometabolic disease and genetic medicines are highly complementary to Verve’s vision and…