LMNA-related dilated cardiomyopathy (LMNA DCM) is one of the most aggressive forms of DCM, affecting approximately 100,000 individuals in the United States and Europe, who progress rapidly to end-stage heart failure. NVC-001 demonstrated significant benefits, including survival and…
Other News
Merck Announces Positive Topline Results From the First Two Phase 3 CORALreef Trials Evaluating Enlicitide Decanoate for the Treatment of Adults With Hyperlipidemia
Enlicitide demonstrated statistically significant and clinically meaningful reductions in LDL-C in both Phase 3 CORALreef HeFH and CORALreef AddOn trials Enlicitide, a novel macrocyclic peptide, has the potential to be the first approved oral PCSK9 inhibitor RAHWAY, N.J.–(BUSINESS WIRE)–Merck (NYSE: MRK), known as MSD outside of the United States and […]
Microbot Medical Continues to Strengthen Commercial Capabilities in Preparation for the anticipated Q3 2025 Launch of its LIBERTY® Endovascular Robotic System
The addition of the head of Sales Operations & Analytics aims to strengthen sales infrastructure and launch execution The addition of the head of Sales Operations & Analytics aims to strengthen sales infrastructure and launch execution
Results from Humacyte’s V007 Pivotal Phase 3 AV Access Study Highlighted by Presentation at the Society for Vascular Surgery Meeting
Benefits of Humacyte’s Acellular Tissue Engineered Vessel Over Autologous Arteriovenous Fistula (AVF) in High-Risk Patients with End-Stage Kidney Disease Observed in Data Presented in Plenary Session Benefits of Humacyte’s Acellular Tissue Engineered Vessel Over Autologous Arteriovenous Fistula (AVF) in High-Risk Patients with End-Stage Kidney Disease Observed in Data Presented in Plenary Session
Microtech Announces the First U.S. Atrial Microsensor Implantations as Part of its FIH Study
TEL AVIV, Israel, June 9, 2025 /PRNewswire/ — Microtech, a wholly owned subsidiary of Medinol, is happy to announce the first U.S. implantations of its atrial-pressure microsensor. Two surgical implantations were performed on Friday, May 16, 2025, at New York-Presbyterian/Columbia…
Pepin Heart Institute at AdventHealth Tampa becomes first in Tampa Bay to complete 1,000th WATCHMAN™ heart procedure
TAMPA, Fla., June 6, 2025 /PRNewswire/ — The heart care experts at the Pepin Heart Institute, a part of AdventHealth Tampa, are now the first in the Tampa Bay area to complete 1,000 WATCHMAN ™ procedures, a minimally-invasive surgery that helps prevent stroke and cardiovascular death in…
Novel Bioengineered Vessel Outperforms Arteriovenous Fistula in High-Risk Patients on Hemodialysis
NEW ORLEANS, LA, JUNE 6, 2025 – Findings from a phase three, randomized controlled clinical trial demonstrate the superiority of Humacyte’s Acellular Tissue Engineered Vessel (ATEV) over autologous arteriovenous fistula (AVF) in high-risk patients with end-stage kidney disease (ESKD). These data highlight the potential of ATEV to address unmet needs […]
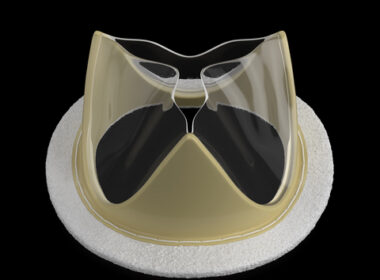
Foldax Secures Approval for TRIA Mitral Heart Valve in India
SALT LAKE CITY–(BUSINESS WIRE)–Foldax® Inc., a leader in heart valve innovation, today announced that the Indian Central Drugs Standard Control Organization (CDSCO) approved its TRIA™ Mitral Valve. Dolphin Life Science India LLP will locally manufacture the TRIA Mitral Valve in India. Foldax’s vision for its novel polymer heart valves is […]
PaceMate® Names JR Finkelmeier CEO As It Sets Its Sights on Large-Scale Expansion
Founding CEO Tripp Higgins To Become Chairman of the Board of Directors, Supporting the Leadership Transition As Finkelmeier Puts Operational Structure in Place to Meet Growing Demand SARASOTA, Fla.–(BUSINESS WIRE)–PaceMate, the cardiac remote monitoring platform that leading healthcare providers trust, today announced the appointment of JR Finkelmeier as Chief Executive […]
Penumbra Introduces the Ruby® XL System – the Longest, Largest, and Softest Coil on the Market for Vascular Embolization
ALAMEDA, Calif., June 5, 2025 /PRNewswire/ — Penumbra, Inc. (NYSE: PEN) announced the U.S. Food and Drug Administration (FDA) clearance and launch of the Ruby® XL System, the longest, largest and softest coil on the marketi. The Ruby XL System is designed to help physicians achieve more…