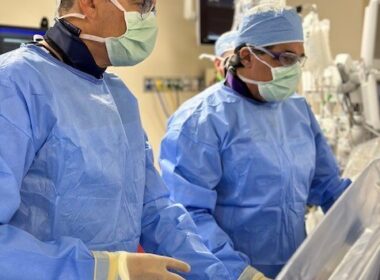
– Commenced market launch and first commercial sales of Symvess™ (acellular tissue engineered vessel-tyod) for the treatment of extremity vascular trauma – – Total revenues of $517,000 for quarter from sales and collaborative research agreement – – Completed public offering raising $46.7 million in net proceeds – – Implemented cost reduction to extend cash runway – —Conference call today at 8:30am ET – DURHAM, N.C., May 13, 2025 (GLOBE NEWSWIRE) — Humacyte, Inc. (Nasdaq: HUMA), a commercial-stage biotechnology platform company developing universally implantable, bioengineered human tissues at commercial scale, today announced financial results for the first quarter ended March 31, 2025, and provided a business update. “The U.S. commercial launch of Symvess this quarter was a major milestone for Humacyte, and we are excited to provide this transformative product to surgeons and patients in need of a new option to save limbs and lives,” said Laura Niklason, M.D., Ph.D., Founder and Chief Executive Officer of Humacyte. “Supporting the launch is our number one priority and we are pleased by the traction gained in our interactions with hospitals, despite the current volatile economic environment. Only a few months after commercial launch, we are excited that 45 hospitals have already commenced an evaluation of Symvess as part of their Value Analysis Committee (VAC) approval process – approximately one quarter of all Level 1 trauma centers nationwide.” “We also appreciate the strong support we have received from surgeons who have treated patients with Symvess, and the resiliency of our team members, in the face of some unfounded negative press regarding Symvess and Humacyte.” continued Dr. Niklason. “Through our March 2025 financing and some recent cost reductions, we have taken steps to extend Humacyte’s cash runway. With this extended runway we will continue to aggressively expand our commercial launch, while creating value from our bioengineering pipeline. Upcoming major value drivers we anticipate include publications of additional clinical results in trauma and in dialysis access, and filing an Investigational New Drug (IND) application with the U.S. Food and Drug Administration (FDA) later this year to enable first-in-human clinical testing of the small-diameter ATEV™ in coronary artery bypass grafting (CABG). As a result of reaching a major enrollment milestone in our V012 Phase 3 trial in dialysis, we are also on track for filing a supplemental Biologics License Application (BLA) for ATEV in dialysis in 2026. First Quarter 2025 and Recent Corporate Highlights Symvess Market Launch First Commercial Sales: Humacyte commenced its commercial launch of Symvess in late February 2025 after the first commercial batch was released by the FDA. The first commercial shipments containing multiple units of Symvess were made during the first quarter to three Level 1 trauma centers.VAC Approval Process: Commencement of sales to hospitals for new products typically requires review and approval by a VAC, which is a centralized decision-making body within each institution. Forty-five hospitals have already initiated the VAC approval process, with additional hospitals expected to commence the process in the near term. The VACs of five hospitals have already approved the purchase of Symvess and we expect this number to increase throughout the second quarter based on current discussions with hospitals.Military Treatment Facilities: Multiple military treatment facilities have expressed interest in purchasing Symvess. To enable these purchases, Humacyte expects shortly to be listed in the Electronic Catalog (ECAT), an internet system that provides the Department of Defense and other Federal agencies with access to manufacturers’ and distributors’ products. Economic Value of Symvess: The Company’s Budget Impact Model was published in March 2025 in the Journal of Medical Economics. Based on the model, the per-patient cost of treating patients with Symvess is estimated to be less than the cost of treating trauma patients with synthetic grafts, cryopreserved allografts, or xenografts. Major drivers of cost savings were attributed to reductions in the rate of amputation and vascular conduit infection.Manufacturing Patent: In January 2025, Humacyte was issued a new U.S. patent covering key aspects of its biomanufacturing platform. The newly issued patent provides protection into 2040 and complements a family of existing patents and patent applications encompassing the design and composition of Symvess and Humacyte’s other product candidates, and their methods of manufacture. ATEV Earlier-Stage Pipeline Major Enrollment Milestone in V012 Phase 3 Study in Dialysis: A total of 84 patients have been enrolled to date in the V012 Phase 3 clinical trial, which is designed to assess the efficacy and safety of the ATEV for dialysis in comparison to arteriovenous (AV) fistulas in female patients. An interim analysis is planned when the first 80 patients reach one-year of follow up, and this enrollment threshold was achieved in April 2025. Subject to these interim results, Humacyte’s plan is to submit a supplemental BLA in the second half of 2026, that includes data from V012 and the V007 Phase 3 pivotal studies, to add AV access for hemodialysis as an indication for the ATEV.Planned IND Filing in CABG: During the quarter Humacyte announced plans to file an IND application with the FDA to enable first-in-human clinical testing of the small-diameter (3.5mm) acellular tissue engineered vessel in CABG. Corporate Update Cost Reduction Actions: In March 2025 Humacyte completed a public offering that provided $46.7 million in net proceeds. In April and May 2025 Humacyte implemented a plan to reduce its workforce by approximately 31 employees, defer additional planned new hires, and reduce other operating expenses. These reductions have been done thoughtfully, and Humacyte has retained key personnel, resources and initiatives to meet our key corporate goals and milestones. Humacyte is undertaking cost reductions to extend its cash runway and to better align its organizational structure with its top business objectives. These objectives include the commercial launch of Symvess including sales, marketing, and manufacturing; completion of the V012 Phase 3 pivotal trial of the ATEV in dialysis and the planned filing of a supplemental BLA with the FDA in the dialysis indication, and; the filing of an IND to commence human study of the small-diameter ATEV in CABG. The Company estimates that it will incur aggregate charges representing one-time cash expenditure for severance and other employee termination benefits of approximately $0.8 million, of which the majority is expected to be incurred during the second quarter of 2025. Humacyte estimates a net savings due to the workforce reductions, operating cost reductions and reduced capital expenditures, net of termination severance and benefits, totaling approximately $13.8 million in 2025. Net savings are estimated to be up to approximately $38.0 million in 2026, for a total estimated savings of over $50 million in 2025 and 2026, relative to original budget forecasts. First Quarter 2025 Financial Highlights There was $517 thousand in revenue for the first quarter of 2025, of which $147 thousand related to the initial U.S. commercial launch of Symvess. The remaining $370 thousand resulted from a research collaboration with a large medical technology company to evaluate the potential use of Humacyte’s bioengineered human tissue in specific cardiovascular and vascular applications. There was no revenue for the first quarter of 2024.Cost of goods sold was $147 thousand for the first quarter of 2025 and includes overhead related to unused production capacity which was recorded as an expense in the period. There was no cost of goods sold for the first quarter of 2024.Research and development expenses for the first quarter of 2025 were $15.4 million compared to $21.3 million for the first quarter of 2024. The decrease in 2025 expenses compared to the prior year period resulted primarily from decreased materials costs as the Company began capitalizing expenditures for inventory during the three months ended March 31, 2025, following the commercial launch of Symvess, as well as a reduction in clinical study costs.General and administrative expenses for the first quarter of 2025 were $8.1 million compared to $5.3 million for the first quarter of 2024. The increase in 2025 expenses compared to the prior year period resulted primarily from the U.S. commercial launch of the Symvess in the vascular trauma indication, including increased personnel expenses.Other net income for the first quarter of 2025 was $62.3 million compared to net expense of $5.3 million for the first quarter of 2024. The increases in 2025 of other net income compared to the prior year period resulted primarily due to an increase in the non-cash remeasurement of the contingent earnout liability associated with the Company’s August 2021 merger with Alpha Healthcare Acquisition Corp.Net income was $39.1 million for the first quarter of 2025 compared to net loss of $31.9 million for the first quarter of 2024. The increase in 2025 net income compared to the prior year period was primarily due to the increase in the non-cash remeasurement of the contingent earnout liability described above.The Company reported cash, cash equivalents and restricted cash of $113.2 million as of March 31, 2025. Total net cash provided was $17.9 million for the first three months of 2025, compared to net cash provided of $35.1 million for the first three months of 2024. The net cash provided for the first three months of 2025 included $46.7 million in net proceeds from a public offering completed in March 2025. The decrease in net cash provided during 2025 compared to the prior year period resulted primarily from the receipt of $20 million in proceeds from a draw under its funding arrangement with Oberland Capital Management in 2024 that did not recur in 2025. Conference Call and Webcast Details Title:Humacyte First Quarter 2025 Financial Results and Corporate UpdateDate:May 13, 2025Time:8:30 AM Eastern TimeConference Call Details:1-877-704-4453 (U.S. Investors Dial) 1-201-389-0920 (International Investors Dial) 13753487 (Conference ID)Call me™Feature:Click HereWebcast:Click Here A replay of the webcast will be available following the conclusion of the live broadcast and will be accessible on the investors section of the Company’s website for at least 30 days. INDICATION Symvess is an acellular tissue engineered vessel indicated for use in adults as a vascular conduit for extremity arterial injury when urgent revascularization is needed to avoid imminent limb loss, and autologous vein graft is not feasible. IMPORTANT SAFETY INFORMATION BOXED WARNING: GRAFT FAILURE Loss of Symvess integrity due to mid-graft rupture or anastomotic failure can result in life threatening hemorrhage. CONTRAINDICATIONS DO NOT use Symvess in patients who have a medical condition that would preclude long-term antiplatelet therapy (such as aspirin or clopidogrel) after resolution of acute injuries. WARNINGS AND PRECAUTIONS Graft Rupture Vascular graft rupture has occurred in patients treated with Symvess. Advise patients that arterial bleeding can be life-threatening and to seek emergent medical evaluation for any signs or symptoms of graft rupture such as bleeding, pain and swelling in the extremity, or signs of extremity ischemia. Anastomotic Failure Anastomotic failure has occurred in patients treated with Symvess. In clinical studies of Symvess, anastomotic failure occurred within the first 36 days post-implantation. Monitor patients for signs of anastomotic failure such as pain and swelling at the surgical site, decreasing hemoglobin or other signs and symptoms of bleeding. Advise patients to seek urgent medical evaluation if they have any signs or symptoms that may be indicative of anastomotic failure such as bleeding, swelling or worsening pain at the surgical site or changes in color of overlying skin. Thrombosis Thrombosis has occurred in patients treated with Symvess. In clinical trials of Symvess, patients received antiplatelet therapy following implantation of Symvess to reduce the risk of thrombosis. The risk of thrombosis may increase in patients who discontinue antiplatelet therapy. Anti-platelet therapy is recommended following treatment with Symvess. Transmission of Infectious Diseases Symvess is manufactured using cells and reagents that may transmit infectious diseases or infectious agents. The cells used in the manufacture of Symvess are derived from a donor who met the donor eligibility requirements for transmissible infectious diseases which includes screening and testing of risks associated with human immunodeficiency virus 1 (HIV-1), human immunodeficiency virus 2 (HIV-2), hepatitis B virus (HBV), hepatitis C virus (HCV), and syphilis (Treponema pallidum). The cell banks are tested negative for human and animal viruses, retroviruses, bacteria, fungi, yeast, and mycoplasma. While all animal-derived reagents are tested for animal viruses, bacteria, fungi, and mycoplasma before use, these measures do not eliminate the risk of transmitting these or other transmissible infectious diseases and disease agents. Fetal bovine serum is sourced to minimize the risk of transmitting a prion protein that causes bovine spongiform encephalopathy and the cause of a rare fatal condition in humans called variant Creutzfeldt-Jakob disease. No transmissible agent infections have been reported during clinical testing. ADVERSE REACTIONS The most common adverse reactions (occurring at ≥ 10%), were vascular graft thrombosis, pyrexia (fever) and pain. Please see full Prescribing Information at www.symvess.com, including Boxed Warning, for Symvess. About Humacyte Humacyte, Inc. (Nasdaq: HUMA) is developing a disruptive biotechnology platform to deliver universally implantable bioengineered human tissues, advanced tissue constructs, and organ systems designed to improve the lives of patients and transform the practice of medicine. The Company develops and manufactures acellular tissues to treat a wide range of diseases, injuries, and chronic conditions. Humacyte’s Biologics License Application for the acellular tissue engineered vessel (ATEV) in the vascular trauma indication was approved by the FDA in December 2024. ATEVs are also currently in late-stage clinical trials targeting other vascular applications, including arteriovenous (AV) access for hemodialysis and peripheral artery disease (PAD). Preclinical development is also underway in coronary artery bypass grafts, pediatric heart surgery, treatment of type 1 diabetes, and multiple novel cell and tissue applications. Humacyte’s 6mm ATEV for AV access in hemodialysis was the first product candidate to receive the FDA’s Regenerative Medicine Advanced Therapy (RMAT) designation and has also received FDA Fast Track designation. Humacyte’s 6mm ATEV for urgent arterial repair following extremity vascular trauma and for advanced PAD also have received RMAT designations. The ATEV received priority designation for the treatment of vascular trauma by the U.S. Secretary of Defense. For more information, visit www.Humacyte.com. For uses other than the FDA approval in the extremity vascular trauma indication, the ATEV is an investigational product and has not been approved for sale by the FDA or any other regulatory agency. Forward-Looking Statements This press release contains forward-looking statements that are based on beliefs and assumptions and on information currently available. In some cases, you can identify forward-looking statements by the following words: “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties, and other factors that may cause actual results, levels of activity, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this press release, we caution you that these statements are based on a combination of facts and factors currently known by us and our projections of the future, about which we cannot be certain. Forward-looking statements in this press release include, but are not limited to, our plans and ability to commercialize Symvess and, if approved by regulatory authorities, our product candidates, successfully and on our anticipated timelines; the degree of market acceptance of and the availability of third-party coverage and reimbursement for Symvess and, if approved by regulatory authorities, our product candidates; our ability to manufacture Symvess and, if approved by regulatory authorities, our product candidates in sufficient quantities to satisfy our clinical trial and commercial needs; the anticipated benefits of our ATEVs relative to existing alternatives; our plans and ability to execute product development, process development and preclinical development efforts successfully and on our anticipated timelines; our ability to design, initiate and successfully complete clinical trials and other studies for our product candidates and our plans and expectations regarding our ongoing or planned clinical trials; the anticipated characteristics and performance of our ATEVs; the implementation of our business model and strategic plans for our business; our ability to execute and achieve the expected benefits of our cost-saving measures and whether our efforts will result in further actions or additional asset impairment charges that adversely affect our business; and the timing or likelihood of regulatory filings, acceptances and approvals. We cannot assure you that the forward-looking statements in this press release will prove to be accurate. These forward-looking statements are subject to a number of significant risks and uncertainties that could cause actual results to differ materially from expected results, including, among others, changes in applicable laws or regulations, the possibility that Humacyte may be adversely affected by other economic, business, and/or competitive factors, and other risks and uncertainties, including those described under the header “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2024, filed by Humacyte with the SEC, and in future SEC filings. Most of these factors are outside of Humacyte’s control and are difficult to predict. Furthermore, if the forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. Except as required by law, we have no current intention of updating any of the forward-looking statements in this press release. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this press release. Humacyte Investor Contact:Joyce AllaireLifeSci Advisors LLC+1-617-435-6602jallaire@lifesciadvisors.com investors@humacyte.com Humacyte Media Contact:Rich LuchettePrecision Strategies+1-202-845-3924rich@precisionstrategies.com media@humacyte.com Humacyte, Inc. Condensed Consolidated Statements of Operations and Comprehensive Income (Loss) (unaudited) (in thousands except for share and per share amounts) Three Months EndedMarch 31, 2025 2024 Revenue: Product revenue, net$147 $— Contract revenue 370 — Total revenue 517 — Operating expenses: Cost of goods sold 147 — Research and development 15,418 21,264 General and administrative 8,136 5,314 Total operating expenses 23,701 26,578 Loss from operations (23,184) (26,578) Other income (expense), net: Change in fair value of contingent earnout liability 49,731 (4,593)Other income (expense) (net) 12,592 (725)Total other income (expense), net 62,323 (5,318)Net income (loss) and comprehensive income (loss)$39,139 $(31,896) Net income (loss) per share, basic$0.28 $(0.29)Weighted-average shares outstanding, basic 131,496,877 108,246,008 Net income (loss) per share, diluted$0.28 $(0.29)Weighted-average shares outstanding, diluted 131,759,302 108,246,008 Humacyte, Inc. Condensed Consolidated Balance Sheets (unaudited) (in thousands) March 31,2025 December 31,2023Assets Current assets: Cash and cash equivalents$62,847 $44,937 Inventory 8,020 — Prepaid expenses and other current assets 2,838 2,922 Total current assets 73,705 47,859 Restricted cash 50,209 50,209 Property and equipment, net 22,436 23,063 Finance lease right-of-use assets, net 14,966 15,490 Other long-term assets 1,237 1,251 Total assets$162,553 $137,872 Liabilities and Stockholders’ Equity (Deficit) Current liabilities: Accounts payable$5,572 $4,490 Accrued expenses 9,701 11,424 Revenue interest liability, current portion 1,690 885 Other current liabilities 3,066 3,155 Total current liabilities 20,029 19,954 Contingent earnout liability 21,230 70,961 Revenue interest liability, net of current portion 64,672 63,354 Finance lease obligation, net of current portion 12,844 13,620 Common stock warrant liabilities 3,320 19,254 Other long-term liabilities 4,415 3,398 Total liabilities 126,510 190,541 Stockholders’ equity (deficit) Common stock and additional paid-in capital 682,919 633,346 Accumulated deficit (646,876) (686,015)Total stockholders’ equity (deficit) 36,043 (52,669)Total liabilities and stockholders’ equity (deficit)$162,553 $137,872
Other News
First Participant Dosed with Acoramidis in ACT-EARLY, the First Ever ATTR Primary Prevention Study
– ATTRibute-CM, BridgeBio’s Phase 3 clinical trial of acoramidis in patients with ATTR-CM, achieved statistical significance in reducing the risk of ACM or first CVH versus placebo in ATTRv-CM patients (59.1% risk reduction), establishing the mechanistic hypothesis that stabilizing TTR may delay or prevent ATTRv-CM – ACT-EARLY is a registrational, randomized, double blind, placebo controlled, event driven prevention study that will enroll ~600 asymptomatic carriers of a pathogenic TTR variant. Diagnosis of ATTRv disease will be evaluated as the primary analysis of the study – In the ACT-EARLY study, BridgeBio will partner globally with ATTR amyloidosis treating physicians and patient advocacy organizations with the hope of addressing a serious unmet need and proving that ATTRv can be delayed or prevented – Acoramidis is approved as Attruby™ by the U.S. FDA and is approved as BEYONTTRA® by the European Commission, Japanese Pharmaceuticals and Medical Devices Agency, and the UK Medicines and Healthcare Products Regulatory Agency PALO ALTO, Calif., May 13, 2025 (GLOBE NEWSWIRE) — BridgeBio Pharma, Inc. (Nasdaq: BBIO) (“BridgeBio” or the “Company”), a new type of biopharmaceutical company focused on genetic diseases, announced today that the first asymptomatic participant with a known pathogenic transthyretin (TTR) variant, that may lead to transthyretin amyloid disease (either cardiomyopathy, ATTR-CM, polyneuropathy, ATTR-PN, or both), has been dosed in ACT-EARLY with acoramidis. Acoramidis is a selective, small molecule, orally administered near-complete (≥90%) TTR stabilizer. “Launching ACT-EARLY is part of our ongoing commitment to further the genetic understanding of the variants causing ATTR and to ensure patients from around the world have access to optimal care. Our hope is that this study will have profound impact to patients and caregivers, and we look forward to growing our partnership with providers and patient advocacy organizations to establish a new prevention paradigm in an area where there is serious unmet need,” said Adam Castaño, M.D., Vice President of Global Clinical Development at BridgeBio Cardiorenal and Head of the ACT-EARLY clinical program. ACT-EARLY is the first ever primary prevention study for ATTR, testing the hypothesis that prophylactic treatment of asymptomatic carriers of a pathogenic TTR variant with the near-complete TTR stabilizer, acoramidis, could delay the onset or prevent the development of variant ATTR (ATTRv), also known as hereditary ATTR (hATTR). ATTRv often presents earlier and progresses more aggressively than the wild-type form of ATTR, leading to significantly worse prognosis. The study aims to randomize ~600 asymptomatic carriers of a pathogenic TTR variant. The primary efficacy endpoint is time to development of ATTR-CM and/or ATTR-PN. Additional endpoints include safety and tolerability of acoramidis, and its effects on cardiac imaging parameters, plasma TTR concentration, nerve conduction and neurofilament light chain. “Current approved therapies for ATTR amyloidosis are only approved to treat diagnosed disease and can only be expected to slow disease progression. There are still many people who carry a genetic variant which puts them at risk of this progressive and fatal disease, and who typically watched other family members suffer through it. Currently, there are no proven prevention treatment options,” said Ahmad Masri, M.D., M.S., Cardiomyopathy Section Head and Director of the Cardiac Amyloidosis Program at Oregon Health & Science University. “By collaborating with BridgeBio on this groundbreaking study, I am hopeful that we can fill the significant gap in care for asymptomatic carriers of a genetic variant by providing potential preventative intervention early with resulting greater clinical benefit than addressing the disease at a later stage.” In ATTR-CM patients, independent of genotype, the ATTRibute-CM Phase 3 trial showed separation at 3 months in time to first event (all-cause mortality (ACM) or cardiovascular-related hospitalization (CVH)) of acoramidis relative to placebo. In a post-hoc analysis, acoramidis led to a 42% reduction in composite ACM and recurrent CVH events relative to placebo at Month 30. Furthermore, acoramidis showed a 50% reduction in the cumulative frequency of CVH events relative to placebo at Month 30. At the American College of Cardiology (ACC) 2025 Annual Scientific Sessions & Expo, BridgeBio disclosed that acoramidis achieved statistical significance in reducing the risk of ACM or first CVH versus placebo in the ATTRv-CM (59.1% risk reduction) subgroup. This treatment effect represents the greatest observed benefit to date for ATTRv-CM patients and establishes the mechanistic hypothesis that stabilization of tetrametric TTR with a near-complete TTR stabilizer, acoramidis, could delay or prevent ATTRv. “I have met many families of those diagnosed with hereditary ATTR and one question often asked is what can be done for asymptomatic carriers of the genetic variant causing ATTR. Since there is currently no approved therapy to delay or prevent disease onset, this underserved, at-risk population must wait for the development of symptoms before therapy can be prescribed,” said Muriel Finkel, President of Amyloidosis Support Groups, a non-profit organization dedicated to the support of amyloidosis patients and caregivers. “I am hopeful that with ACT-EARLY, loved ones of those with variant ATTR will be able to get genetic testing done, and if they meet the qualification criteria, can get started on a clinical trial that might identify whether prophylactic treatment will slow down or prevent ATTR at its genetic source.” Acoramidis is approved as Attruby by the U.S. FDA and is approved as BEYONTTRA by the European Commission, Japanese Pharmaceuticals and Medical Devices Agency, and UK Medicines and Healthcare Products Regulatory Agency with all labels specifying near-complete stabilization of TTR. ACT-EARLY (NCT06563895) is currently enrolling participants. More information on the study can be found at ACTEARLY.com. About Attruby™ (acoramidis) INDICATION Attruby is a transthyretin stabilizer indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization. IMPORTANT SAFETY INFORMATION Adverse Reactions Diarrhea (11.6% vs 7.6%) and upper abdominal pain (5.5% vs 1.4%) were reported in patients treated with Attruby versus placebo, respectively. The majority of these adverse reactions were mild and resolved without drug discontinuation. Discontinuation rates due to adverse events were similar between patients treated with Attruby versus placebo (9.3% and 8.5%, respectively). About Pharma, Inc.BridgeBio Pharma, Inc. (BridgeBio) is a new type of biopharmaceutical company founded to discover, create, test and deliver transformative medicines to treat patients who suffer from genetic diseases and cancers with clear genetic drivers. BridgeBio’s pipeline of development programs ranges from early science to advanced clinical trials. BridgeBio was founded in 2015 and its team of experienced drug discoverers, developers, and innovators are committed to applying advances in genetic medicine to help patients as quickly as possible. For more information visit bridgebio.com and follow us on LinkedIn, Twitter, Facebook, and YouTube. BridgeBio Forward-Looking Statements This press release contains forward-looking statements. Statements in this press release may include statements that are not historical facts and are considered forward-looking within the meaning of Section 27A of the Securities Act of 1933, as amended (the Securities Act), and Section 21E of the Securities Exchange Act of 1934, as amended (the Exchange Act), which are usually identified by the use of words such as “anticipates,” “believes,” “continues,” “estimates,” “expects,” “hopes,” “intends,” “may,” “plans,” “projects,” “remains,” “seeks,” “should,” “will,” and variations of such words or similar expressions. BridgeBio intends these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Exchange Act. These forward-looking statements, including the potential for the stabilization of tetrametric TTR to delay the onset or prevent the development of ATTR(v), the expected enrollment of ~600 asymptomatic carriers of a pathogenic TTR variant in the ACT-EARLY study, the Company’s expectation to partner globally with ATTR amyloidosis treating physicians and patient advocacy organizations in the ACT-EARLY study, the progress of the ACT-EARLY study, and the potential for the ACT-EARLY study to achieve its endpoints, provide preventative intervention to asymptomatic carriers of ATTR amyloidosis and impact patients and caregivers, among others, reflect BridgeBio’s current views about its plans, intentions, expectations and strategies, which are based on the information currently available to BridgeBio and on assumptions BridgeBio has made. Although BridgeBio believes that its plans, intentions, expectations and strategies as reflected in or suggested by those forward-looking statements are reasonable, BridgeBio can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a number of risks, uncertainties and assumptions, including, but not limited to the risks associated with BridgeBio’s dependence on third parties for development, the risks regulatory authorities may require additional studies or data to support the continued or expanded commercialization of acoramidis, data and results may not meet regulatory requirements or otherwise be sufficient for further development, regulatory review, approval or continued commercialization, other regulatory agencies not agreeing with BridgeBio’s regulatory approval strategies, components of BridgeBio’s filings, such as clinical trial designs, conduct and methodologies, or the sufficiency of data submitted, the continuing success of its collaborations, and uncertainty regarding any impacts due to global health emergencies, including delays in regulatory reviews and other regulatory activities, manufacturing and supply chain interruptions, adverse effects on healthcare systems and disruption of the global economy, the impacts of current macroeconomic and geopolitical events, including changing conditions from hostilities in Ukraine and in Israel and the Gaza Strip, increasing rates of inflation and changing interest rates, on BridgeBio’s business operations and expectations, as well as those risks set forth in the Risk Factors section of BridgeBio’s most recent Annual Report on Form 10-K and Quarterly Report on From 10-Q and its other filings with the U.S. Securities and Exchange Commission. Moreover, BridgeBio operates in a very competitive and rapidly changing environment in which new risks emerge from time to time. These forward-looking statements are based upon the current expectations and beliefs of BridgeBio’s management as of the date of this press release and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Except as required by applicable law, BridgeBio assumes no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. BridgeBio Media Contact:Bubba Murarka, EVP Communicationscontact@bridgebio.com(650)-789-8220 BridgeBio Investor Contact:Chinmay Shukla, VP IR & Strategic FinanceChinmay.shukla@bridgebio.com
Catheter Precision, Inc. Announces $1.5 Million Private Placement Equity Financing and Potential Strategic Alliance
Fort Mill, S.C., May 12, 2025 (GLOBE NEWSWIRE) — Catheter Precision (VTAK – NYSE/American), a US based medical device company focused on developing technologically advanced products for the cardiac electrophysiology market, today announced that it has entered into securities purchase agreements with institutional investors for a $1.5 million private placement equity financing and the acquisition of certain promissory notes of QHSLab, Inc.
Orchestra BioMed Reports First Quarter 2025 Financial Results and Highlights Recent Regulatory and Clinical Milestones
FDA Breakthrough Device Designation Awarded to AVIM Therapy FDA IDE Approved for Virtue SAB U.S. Pivotal Trial for Launch NEW HOPE, Pa., May 12, 2025 (GLOBE NEWSWIRE) — Orchestra BioMed Holdings, Inc. (Nasdaq: OBIO, “Orchestra BioMed” or the “Company”), a biomedical company accelerating high-impact technologies to patients through risk-reward sharing partnerships, today announced financial results for the first quarter ended March 31, 2025, and provided a business update highlighting continued regulatory momentum, disciplined operational execution, and a strengthening clinical development pipeline. Q1 2025 Highlights: U.S. Food and Drug Administration (“FDA”) Breakthrough Device Designation (“BDD”) granted for atrioventricular interval modulation (“AVIM”) therapy in patients with uncontrolled hypertension and increased cardiovascular risk, with or without an indication for a pacemaker, marking a major regulatory validation of the therapy’s potential to improve hypertensive heart disease outcomes. FDA BDD enables prioritized regulatory interactions and strengthens potential access to add-on reimbursement.BACKBEAT Global Pivotal Study execution continuing to progress in strategic collaboration with Medtronic (NYSE: MDT).New Clinical Data presented demonstrating the favorable impact of AVIM therapy on cardiac function in patients with diastolic dysfunction, a key contributor to the development of heart failure, as late breaker at THT 2025.Intellectual Property Expansion continues with AVIM therapy patent estate reaching 137 issued patents worldwide, with recent additions bolstering coverage for both hypertension and heart failure indications.FDA Investigational Device Exemption (“IDE”) Approval received for the Virtue® Sirolimus AngioInfusion Balloon (“SAB”) U.S. pivotal trial, a randomized head-to-head study comparing Virtue SAB with the commercially available AGENTTM DCB paclitaxel-coated balloon (the “Virtue Trial”). Trial initiation is currently targeted for the second half of 2025. Orchestra BioMed is sponsoring and in full operational control of the Virtue Trial; mediation with Terumo of certain other contractual terms is progressing. Key Chief Executive Officer (“CEO”) Commentary: David Hochman, Chairman and CEO of Orchestra BioMed, stated: “We continued to make meaningful progress during the first quarter with significant inflection points for both our AVIM therapy and Virtue SAB programs. We believe the FDA’s Breakthrough Device Designation for AVIM therapy signals meaningful recognition of its potential to meet the clinical needs of millions living with hypertensive heart disease, a population in which chronic high blood pressure can lead to various heart problems like left ventricular hypertrophy, diastolic dysfunction, heart failure, and coronary artery disease. We believe that AVIM therapy has the potential to provide a potent additional therapeutic option for these patients. We are focused on execution of the BACKBEAT global pivotal study alongside our strategic partner Medtronic as the critical pathway toward making AVIM therapy available to patients globally.” Mr. Hochman continued, “In parallel, we secured full IDE approval to conduct the Virtue Trial, a U.S. pivotal coronary study that will evaluate Virtue SAB, our investigational, non-coated drug-eluting balloon designed to deliver a large liquid dose of proprietary extended-release sirolimus, head-to-head against the commercially available AGENT paclitaxel-coated balloon. We believe the updated design of the trial, which we currently plan to initiate in the second half of the year, offers the most direct path to regulatory approval while showcasing the distinctive pharmacokinetic and therapeutic advantages of our proprietary technology. Taken together, these milestones mark tangible progress on multiple fronts. We continue to execute methodically and remain focused on advancing our pivotal trials, generating clinical evidence, and building long-term value for patients, physicians, shareholders and strategic partners alike.” Financial Results for the First Quarter Ended March 31, 2025 Cash and cash equivalents and Marketable securities totaled $49.9 million as of March 31, 2025.Net cash used in operating activities and for the purchase of fixed assets was $16.7 million during the first quarter of 2025, compared with $13.1 million for the first quarter in 2024, with the primary driver being increased research and development costs during the first quarter of 2025.Revenue for the first quarter of 2025 was $0.9 million, compared with $0.6 million for the first quarter in 2024. The increase was primarily due to recognition of partnership revenues earned under the agreement with Terumo.Research and development expenses for the first quarter of 2025 were $13.5 million, compared with $9.1 million for the first quarter in 2024. The increase was primarily due to additional costs associated with the ongoing BACKBEAT global pivotal study.Selling, general and administrative expenses for the first quarter of 2025 were $6.3 million, compared with $5.9 million for the first quarter of 2024. The increase was primarily due to an increase in stock-based compensation and increased professional fees associated with the overall growth of the business.Net loss for the first quarter of 2025 was $18.8 million, or $0.49 per share, compared with a net loss of $13.5 million, or $0.38 per share, for the first quarter of 2024. Net loss for the first quarter of 2025 included $3.0 million in non-cash stock-based compensation expense as compared to $2.6 million for the same period in 2024. About Orchestra BioMed Orchestra BioMed (Nasdaq: OBIO) is a biomedical innovation company accelerating high-impact technologies to patients through risk-reward sharing partnerships with leading medical device companies. Orchestra BioMed’s partnership-enabled business model focuses on forging strategic collaborations with leading medical device companies to drive successful global commercialization of products it develops. Orchestra BioMed’s lead product candidate is AVIM therapy for the treatment of hypertension, the leading risk factor for death worldwide. Orchestra BioMed is also developing Virtue SAB for the treatment of atherosclerotic artery disease, the leading cause of mortality worldwide. Orchestra BioMed has a strategic collaboration with Medtronic, one of the largest medical device companies in the world, for development and commercialization of AVIM therapy for the treatment of hypertension in pacemaker-indicated patients, and a strategic partnership with Terumo, a global leader in medical technology, for development and commercialization of Virtue SAB for the treatment of artery disease. The Company has received four Breakthrough Device Designations from the U.S. FDA across these two core programs, reflecting the significant potential of its technologies to address high unmet needs in cardiovascular care. For further information about Orchestra BioMed, please visit www.orchestrabiomed.com, and follow us on LinkedIn. References to Websites and Social Media Platforms References to information included on, or accessible through, websites and social media platforms do not constitute incorporation by reference of the information contained at or available through such websites or social media platforms, and you should not consider such information to be part of this press release. About AVIM Therapy AVIM therapy is an investigational therapy compatible with standard dual-chamber pacemakers designed to substantially and persistently lower blood pressure. It has been evaluated in pilot studies in patients with hypertension who are also indicated for a pacemaker. MODERATO II, a double-blind, randomized pilot study, showed that patients treated with AVIM therapy experienced net reductions of 8.1 mmHg in 24-hour ambulatory systolic blood pressure (aSBP) and 12.3 mmHg in office systolic blood pressure (oSBP) at six months when compared to control patients. In addition to reducing blood pressure, clinical results using AVIM therapy demonstrate improvements in cardiac function and hemodynamics. The BACKBEAT (BradycArdia paCemaKer with atrioventricular interval modulation for Blood prEssure treAtmenT) global pivotal study will further evaluate the safety and efficacy of AVIM therapy in lowering blood pressure in patients who have systolic blood pressure above target despite anti-hypertensive medication and who are indicated for or have recently received a dual-chamber cardiac pacemaker. AVIM therapy has been granted Breakthrough Device Designation by the FDA for the treatment of uncontrolled hypertension in patients who have increased cardiovascular risk. About Virtue SAB Virtue SAB is an investigational therapeutic combination drug-device designed to deliver a proprietary extended-release formulation of sirolimus, SirolimusEFR™ through a non-coated microporous AngioInfusion™ Balloon, protecting the drug in transit through the arteries and consistently delivering a large liquid dose, overcoming certain limitations of drug-coated balloons. SirolimusEFR delivered by Virtue SAB has been shown in published preclinical series involving hundreds of arterial deliveries to achieve sustained tissue levels well above the known required therapeutic tissue concentration for inhibiting restenosis (1 ng/mg tissue) for the entire critical healing period of approximately 30 days. Virtue SAB demonstrated positive three-year clinical data in coronary in-stent restenosis (“ISR”) in the SABRE study, a multi-center prospective, independent core lab-adjudicated clinical study conducted in Europe. Virtue SAB has been granted Breakthrough Device Designation by the FDA for specific indications relating to coronary ISR, coronary small vessel disease and peripheral artery disease below-the-knee. The FDA granted IDE approval for Orchestra BioMed to evaluate the efficacy and safety of Virtue SAB in the Virtue Trial, a pivotal trial that will randomize Virtue SAB against commercially available AGENTTM DCB, a paclitaxel-coated balloon. Forward-Looking Statements Certain statements included in this press release that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements relating to the initiation, enrollment, timing, implementation and design of the Company’s planned and ongoing pivotal trials and reporting of top-line results, including the timing of initiation of the Virtue Trial, the potential benefits of BDD, including its ability to expedite FDA reviews and allow access to add-on reimbursement, realizing the clinical and commercial value of AVIM therapy and Virtue SAB, the potential safety and efficacy of the Company’s product candidates, and the ability of the Company’s partnerships to accelerate clinical development. These statements are based on various assumptions, whether or not identified in this press release, and on the current expectations of the Company’s management and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as and must not be relied on as a guarantee, an assurance, a prediction, or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and may differ from assumptions. Many actual events and circumstances are beyond the control of the Company. These forward-looking statements are subject to a number of risks and uncertainties, including changes in domestic and foreign business, market, financial, political, and legal conditions; risks related to regulatory approval of the Company’s commercial product candidates and ongoing regulation of the Company’s product candidates, if approved; the timing of, and the Company’s ability to achieve expected regulatory and business milestones; the impact of competitive products and product candidates; and the risk factors discussed under the heading “Item 1A. Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, which was filed with the SEC on March 31, 2025 and the risk factor discussed under the heading “Item 1A. Risk Factors” in the Company’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2025, which was filed with the SEC on May 12, 2025. The Company operates in a very competitive and rapidly changing environment. New risks emerge from time to time. Given these risks and uncertainties, the Company cautions against placing undue reliance on these forward-looking statements, which only speak as of the date of this press release. The Company does not plan and undertakes no obligation to update any of the forward-looking statements made herein, except as required by law. Investor ContactSilas NewcombOrchestra BioMed Snewcomb@orchestrabiomed.com Media ContactKelsey Kirk-EllisOrchestra BioMedKkirkellis@orchestrabiomed.com ORCHESTRA BIOMED HOLDINGS, INC.Condensed Consolidated Balance Sheets(in thousands, except share and per share data)(Unaudited) March 31, December 31, 2025 2024ASSETS CURRENT ASSETS: Cash and cash equivalents $18,348 $22,261 Marketable securities 31,536 44,551 Accounts receivable, net 89 92 Inventory 135 173 Prepaid expenses and other current assets 1,795 2,094 Total current assets 51,903 69,171 Property and equipment, net 1,413 1,384 Right-of-use assets 1,956 2,103 Strategic investments, less current portion 2,495 2,495 Deposits and other assets 1,284 1,020 TOTAL ASSETS $59,051 $76,173 LIABILITIES AND STOCKHOLDERS’ EQUITY CURRENT LIABILITIES: Accounts payable $5,563 $5,134 Accrued expenses and other liabilities 5,392 6,084 Operating lease liability, current portion 590 550 Deferred revenue, current portion 3,944 4,439 Total current liabilities 15,489 16,207 Deferred revenue, less current portion 10,752 10,989 Loan payable 14,338 14,292 Operating lease liability, less current portion 1,516 1,687 Other long-term liabilities 99 40 TOTAL LIABILITIES 42,194 43,215 STOCKHOLDERS’ EQUITY Preferred stock, $0.0001 par value per share; 10,000,000 shares authorized; none issued or outstanding at March 31, 2025 and December 31, 2024. — — Common stock, $0.0001 par value per share; 340,000,000 shares authorized; 38,312,512 and 38,194,442 shares issued and outstanding as of March 31, 2025 and December 31, 2024, respectively. 4 4 Additional paid-in capital 345,449 342,780 Accumulated other comprehensive income 37 52 Accumulated deficit (328,633) (309,878)TOTAL STOCKHOLDERS’ EQUITY 16,857 32,958 TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY $59,051 $76,173 ORCHESTRA BIOMED HOLDINGS, INC.Condensed Consolidated Statements of Operations and Comprehensive Loss(in thousands, except share and per share data)(Unaudited) Three Months Ended March 31, 2025 2024 Revenue: Partnership revenue $732 $497 Product revenue 136 123 Total revenue 868 620 Expenses: Cost of product revenues 44 34 Research and development 13,482 9,112 Selling, general and administrative 6,263 5,897 Total expenses 19,789 15,043 Loss from operations (18,921) (14,423)Other income (expense): Interest income, net 166 1,016 Loss on fair value of strategic investments — (45)Other expense — (11)Total other income 166 960 Net loss $(18,755) $(13,463)Net loss per share Basic and diluted $(0.49) $(0.38)Weighted-average shares used in computing net loss per share, basic and diluted 38,235,409 35,777,877 Comprehensive loss Net loss $(18,755) $(13,463)Unrealized (loss) gain on marketable securities (15) 2 Comprehensive loss $(18,770) $(13,461)
Mineralys Therapeutics Reports First Quarter 2025 Financial Results and Provides Corporate Update
– Pivotal Advance-HTN and Launch-HTN trials successfully achieved statistical significance in primary efficacy endpoints and demonstrated a favorable safety and tolerability profile – – Anticipate Explore-CKD Phase 2 trial to deliver topline data in Q2 2025 – – Initiated Explore-OSA Phase 2 Trial in Q1 2025 – – Conference call today at 4:30 p.m. ET – RADNOR, Pa., May 12, 2025 (GLOBE NEWSWIRE) — Mineralys Therapeutics, Inc. (Nasdaq: MLYS), a clinical-stage biopharmaceutical company focused on developing medicines to target hypertension, chronic kidney disease (CKD), obstructive sleep apnea (OSA) and other diseases driven by dysregulated aldosterone, today announced financial results for the first quarter ended March 31, 2025, and provided a corporate update. “We are pleased to have recently announced positive topline results from our pivotal trials, Launch-HTN and Advance-HTN, that evaluated lorundrostat’s efficacy and safety as a potential treatment for patients with uncontrolled or resistant hypertension. The Advance-HTN late-breaking presentation at the American College of Cardiology meeting and the recent publication in the New England Journal of Medicine underscore the strength of our clinical data and the potentially transformative nature of lorundrostat. With the success of our two pivotal trials, we are working toward submitting our new drug application with a pre-NDA meeting with the FDA anticipated in the fourth quarter of 2025,” stated Jon Congleton, Chief Executive Officer of Mineralys Therapeutics. “We are excited to announce the appointment of Eric Warren as our Chief Commercial Officer. Eric’s proven leadership skills, extensive cardiovascular experience and track record of success, will be invaluable to Mineralys as we solidify our commercial and partnering strategy.” Recent Clinical Highlights and Upcoming Milestones Pivotal Launch-HTN Phase 3 Trial – The trial met its primary endpoint in evaluating the efficacy and safety of lorundrostat for the treatment of subjects with uncontrolled hypertension (uHTN) or resistant hypertension (rHTN) as add-on therapy, who fail to achieve blood pressure control on their existing medications. The trial reported that the lorundrostat 50 mg dose achieved a 16.9 mmHg reduction in systolic blood pressure, and a 9.1 mmHg placebo-adjusted reduction (p-value < 0.0001), as assessed by automated office blood pressure at week six. This benefit was sustained with potential further reduction through week 12, with a 19.0 mmHg reduction in automated office systolic blood pressure and an 11.7 mmHg placebo-adjusted reduction (p-value 6.0 mmol/L) at the scheduled study visit was 1.1% and 1.5% in the 50 mg and 50 to 100 mg arms, respectively. After exclusion of factitious test results, the incidence of confirmed hyperkalemia was 0.6% and 1.1%, respectively. The Launch-HTN trial has been accepted as a late-breaking presentation at the 2025 European Society of Hypertension Meeting on Hypertension and Cardiovascular Protection, which is being held in Milan, Italy on May 23-26, 2025. Pivotal Advance-HTN Trial – Detailed results from the Advance-HTN trial were recently presented in a late-breaking presentation at the American College of Cardiology’s ACC.25 meeting and published on May 8th in the New England Journal of Medicine. The trial met its primary endpoints in evaluating the efficacy and safety of lorundrostat for the treatment of confirmed uHTN or rHTN. The trial reported that the lorundrostat 50 mg dose cohort achieved a 15.4 mmHg absolute reduction in systolic blood pressure and a 7.9 mmHg placebo-adjusted reduction (p-value = 0.001), as assessed by 24-hour ambulatory blood pressure monitoring at 12 weeks. Lorundrostat demonstrated a favorable safety and tolerability profile, with modest changes in serum potassium, serum sodium and eGFR, and a low discontinuation rate. The incidence of hyperkalemia (serum potassium >6.0 mmol/L) at the scheduled study visit was 5.3% and 7.4% in the 50 mg and 50 to 100 mg arms, respectively. After exclusion of factitious test results, the incidence of confirmed hyperkalemia was 2.1% and 3.2%, respectively. These results reinforce lorundrostat’s favorable benefit-risk profile in a high-risk population that would typically be treated by specialists rather than general practitioners.Transform-HTN Open-Label Extension Trial – The Company’s ongoing open-label extension trial allows subjects to continue to receive lorundrostat and the Company to obtain additional safety and efficacy data.Explore-CKD Phase 2 Trial – Enrollment has been completed and topline data are anticipated in the second quarter of 2025. The trial is designed to evaluate the safety and efficacy of lorundrostat when added to background treatment with an ACE inhibitor or ARB and a SGLT2 inhibitor for the treatment of hypertension in subjects with Stage 2 to 3b CKD (eGFR greater than or equal to 30 mL/min/1.73m2 ) and albuminuria.Explore-OSA Phase 2 Trial – The Company initiated the trial in the first quarter of 2025, which will evaluate the safety and efficacy of lorundrostat in the treatment of overweight and obese subjects with moderate-to-severe OSA and hypertension.Expanded Management Team – Appointed Eric Warren as Chief Commercial Officer. Mr. Warren brings more than three decades of commercial leadership experience across multiple healthcare segments with a heavy emphasis on cardiovascular disease. Mr. Warren will lead the Company’s commercial strategy and support future partnering opportunities.Strengthened Balance Sheet – On March 18, 2025, the Company completed a public equity financing for gross proceeds of approximately $201.2 million, before deducting fees and expenses. The Company reported a total of $343.0 million of cash, cash equivalents and investments as of March 31, 2025. First Quarter 2025 Financial Highlights Cash, cash equivalents and investments were $343.0 million as of March 31, 2025, compared to $198.2 million as of December 31, 2024. The Company believes that its current cash, cash equivalents and investments will be sufficient to fund its planned clinical trials and regulatory activities, as well as support corporate operations, into 2027. Research and Development (R&D) expenses for the quarter ended March 31, 2025 were $37.9 million, compared to $30.8 million for the quarter ended March 31, 2024. The increase in R&D expenses was primarily due to increases of $4.8 million in preclinical and clinical costs and $2.8 million in compensation expense resulting from additions to headcount, increases in salaries and accrued bonuses and increased stock-based compensation, partially offset by $0.5 million in lower clinical supply, manufacturing and regulatory costs. General and Administrative (G&A) expenses were $6.6 million for the quarter ended March 31, 2025, compared to $4.6 million for the quarter ended March 31, 2024. The increase in G&A expenses was primarily due to $1.2 million in higher compensation expense resulting from additions to headcount, increases in salaries and accrued bonuses and increased stock-based compensation, and $0.7 million in higher professional fees. Total other income, net was $2.2 million for the quarter ended March 31, 2025, compared to $3.9 million for the quarter ended March 31, 2024. The decrease was primarily attributable to decreased interest earned on our investments in money market funds and U.S. treasuries. Net loss was $42.2 million for the quarter ended March 31, 2025, compared to $31.5 million for the quarter ended March 31, 2024. The increase was primarily attributable to the factors impacting the Company’s expenses described above. Conference Call The Company’s management team will host a conference call at 4:30 p.m. ET on Monday, May 12, 2025. To access the call, please dial 1-800-717-1738 in the United States or 1-646-307-1865 outside the United States. A live webcast of the conference call may be found here. A replay of the call will be available on the “News & Events” page in the Investor Relations section of the Mineralys Therapeutics website (click here). About Hypertension Having sustained, elevated blood pressure (or hypertension) increases the risk of heart disease, heart attack and stroke, which are leading causes of death in the United States. In 2022, more than 685,000 deaths in the United States included hypertension as a primary or contributing cause. Hypertension and related health issues resulted in an average annual economic burden of about $219 billion in the United States in 2019. Less than 50% of hypertension patients achieve their blood pressure goal with currently available medications. Dysregulated aldosterone levels are a key factor in driving hypertension in approximately 30% of all hypertensive patients. About CKD CKD, which is characterized by the gradual loss of kidney function, is estimated to affect more than 10% of the global population and is one of the leading causes of mortality worldwide. According to the U.S. Centers for Disease Control and Prevention (CDC), an estimated 1-in-7 (15%) of U.S. adults have CKD. Diabetes and hypertension are responsible for approximately two-thirds of CKD cases. Early detection and treatment can often keep CKD from getting worse. When CKD progresses, it may eventually lead to kidney failure, which requires dialysis or a kidney transplant to maintain life. About OSA OSA is characterized by repetitive overnight hypoxic episodes and subsequent sleep fragmentation due to a complete or partial collapse of the upper airway. Moderate OSA is defined as having between 15 and 30 breathing pauses (apnea or hypopnea events) per hour of sleep, while severe OSA indicates more than 30 breathing pauses per hour. OSA impacts almost one billion people globally, including 425 million moderate-to-severe cases. Around 80% of adults with OSA are undiagnosed. As of 2015, undiagnosed OSA is estimated to cost the United States approximately $149.6 billion annually from comorbid disease, workplace accidents, motor vehicle accidents and loss of workplace productivity. Between 30-50% of adults with hypertension have OSA, and this number increases to between 70-80% in adults with rHTN. Additionally, untreated moderate-to-severe OSA increases the risk of rHTN. Along with hypertension, OSA is a major risk factor of cardiovascular disease, type-2 diabetes mellitus and stroke. About Lorundrostat Lorundrostat is a proprietary, orally administered, highly selective aldosterone synthase inhibitor being developed for the treatment of uHTN or rHTN, as well as CKD and OSA. Lorundrostat was designed to reduce aldosterone levels by inhibiting CYP11B2, the enzyme responsible for its production. Lorundrostat has 374-fold selectivity for aldosterone-synthase inhibition versus cortisol-synthase inhibition in vitro, an observed half-life of 10-12 hours and demonstrated a 40-70% reduction in plasma aldosterone concentration in hypertensive subjects. In a Phase 2, proof-of-concept trial (Target-HTN) in uncontrolled or resistant hypertensive subjects, once-daily lorundrostat demonstrated statistically significant and clinically meaningful systolic blood pressure reduction in both automated office systolic blood pressure measurement and 24-hour ambulatory systolic blood pressure monitoring. Adverse events observed were a modest increase in serum potassium, decrease in eGFR, urinary tract infection and hypertension, with one serious adverse event possibly related to study drug being hyponatremia. About Mineralys Mineralys Therapeutics is a clinical-stage biopharmaceutical company focused on developing medicines to target hypertension, CKD, OSA and other diseases driven by dysregulated aldosterone. Its initial product candidate, lorundrostat, is a proprietary, orally administered, highly selective aldosterone synthase inhibitor that Mineralys Therapeutics is developing for the treatment of cardiorenal conditions affected by dysregulated aldosterone, including hypertension, CKD and OSA. Mineralys is based in Radnor, Pennsylvania, and was founded by Catalys Pacific. For more information, please visit https://mineralystx.com. Follow Mineralys on LinkedIn and Twitter. Forward-Looking Statements Mineralys Therapeutics cautions you that statements contained in this press release regarding matters that are not historical facts are forward-looking statements. The forward-looking statements are based on our current beliefs and expectations and include, but are not limited to, statements regarding: the potential therapeutic benefits of lorundrostat; the Company’s expectation that aldosterone synthase inhibitors with an SGLT2 inhibitor may provide additive clinical benefits to patients; the Company’s expectation that Advance-HTN and Launch-HTN may serve as pivotal trials in any submission of a new drug application (NDA) to the U.S. Food and Drug Administration (FDA); the Company’s ability to evaluate lorundrostat as a potential treatment for CKD, OSA, uHTN or rHTN; the planned future clinical development of lorundrostat and the timing thereof; and the expected timing of commencement and enrollment of patients in clinical trials and topline results from clinical trials. Actual results may differ from those set forth in this press release due to the risks and uncertainties inherent in our business, including, without limitation: topline results that we report are based on a preliminary analysis of key efficacy and safety data, and such data may change following a more comprehensive review of the data related to the clinical trial and such topline data may not accurately reflect the complete results of a clinical trial; our future performance is dependent entirely on the success of lorundrostat; potential delays in the commencement, enrollment and completion of clinical trials and nonclinical studies; later developments with the FDA may be inconsistent with the feedback from the completed end of Phase 2 meeting, including whether the proposed pivotal program will support registration of lorundrostat which is a review issue with the FDA upon submission of an NDA; the results of our clinical trials, including the Advance-HTN and Launch-HTN trials, may not be deemed sufficient by the FDA to serve as the basis for an NDA submission or regulatory approval of lorundrostat; our dependence on third parties in connection with manufacturing, research and clinical and nonclinical testing; unexpected adverse side effects or inadequate efficacy of lorundrostat that may limit its development, regulatory approval and/or commercialization; unfavorable results from clinical trials and nonclinical studies; results of prior clinical trials and studies of lorundrostat are not necessarily predictive of future results; macroeconomic trends and uncertainty with regard to high interest rates, elevated inflation, tariffs, and the potential for a local and/or global economic recession; our ability to maintain undisrupted business operations due to any pandemic or future public health concerns; regulatory developments in the United States and foreign countries; our reliance on our exclusive license with Mitsubishi Tanabe Pharma to provide us with intellectual property rights to develop and commercialize lorundrostat; and other risks described in our filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in our annual report on Form 10-K, and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and we undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Contact:Investor Relationsinvestorrelations@mineralystx.com Media RelationsTom WeibleElixir Health Public RelationsPhone: (1) 515-707-9678Email: tweible@elixirhealthpr.com Mineralys Therapeutics, Inc.Condensed Statements of Operations(in thousands, except share and per share data)(unaudited) Three Months Ended March 31, 2025 2024 Operating expenses: Research and development $37,879 $30,754 General and administrative 6,568 4,608 Total operating expenses 44,447 35,362 Loss from operations (44,447) (35,362)Interest income, net 2,239 3,853 Other income (expense) (3) 1 Total other income, net 2,236 3,854 Net loss $(42,211) $(31,508)Net loss per share attributable to common stockholders, basic and diluted $(0.79) $(0.70)Weighted-average shares used to compute net loss per share attributable to common stockholders, basic and diluted 53,163,551 44,900,755 Mineralys Therapeutics, Inc.Selected Financial InformationCondensed Balance Sheet Data(amounts in thousands)(unaudited) March 31, December 31, 2025 2024 Cash, cash equivalents and investments $343,026 $198,187 Total assets $354,941 $205,903 Total liabilities $13,386 $14,646 Total stockholders’ equity $341,555 $191,257
Medera to Present Late-Breaking Phase 1/2a Clinical Trial Results at Heart Failure 2025 Congress
BOSTON, May 12, 2025 (GLOBE NEWSWIRE) — Medera Inc. (“Medera”), a clinical-stage biopharmaceutical company focused on targeting cardiovascular diseases by developing a range of next-generation therapeutics, today announced that data from its First-In-Human Phase 1/2a MUSIC-HFpEF clinical trial investigating its adeno-associated virus-based gene therapy candidate SRD-002 in heart failure with preserved ejection fraction (HFpEF) will be presented at the upcoming Heart Failure 2025 Congress taking place May 17-20, 2025, in Belgrade, Serbia.
Windtree Therapeutics Announces Presentation of Preclinical Data on Istaroxime and a Selective SERCA2a Activator at the European Society of Cardiology Heart Failure Conference May 17, 2025
ePoster presentation advances the preclinical evaluation of the importance of SERCA2a function to reduce arrythmias with istaroxime and a selective SERCA2a activator in the setting of ischemia and reperfusion ePoster presentation advances the preclinical evaluation of the importance of SERCA2a function to reduce arrythmias with istaroxime and a selective SERCA2a activator in the setting of ischemia and reperfusion
Lexeo Therapeutics Reports First Quarter 2025 Financial Results and Operational Highlights
Announced positive interim data for LX2006 from Phase 1/2 studies in Friederich ataxia (FA) cardiomyopathy; frataxin expression and LVMI improvement exceeded co-primary target thresholds for planned registrational study LX2006 registrational study expected to begin by early 2026; commencing enrollment in prospective natural history study, CLARITY-FA, in Q2 2025 to serve as concurrent external control Phase 1/2 clinical trial of LX2020 (HEROIC-PKP2) currently enrolling patients in Cohort 3; interim clinical data update on track for second half of 2025 Redeployed $20 million to focus on clinical-stage programs; cash, cash equivalents and investments of $106.9 million expected to provide operational runway into 2027 NEW YORK, May 12, 2025 (GLOBE NEWSWIRE) — Lexeo Therapeutics, Inc. (Nasdaq: LXEO), a clinical stage genetic medicine company dedicated to pioneering novel treatments for cardiovascular diseases, today provided business updates across its portfolio and reported first quarter 2025 financial results. “Based on the highly encouraging clinical data shared to date, we believe LX2006 could be transformational and establish a new standard of care in FA cardiomyopathy,” said R. Nolan Townsend, Chief Executive Officer of Lexeo Therapeutics. “We look forward to beginning enrollment in the CLARITY-FA natural history study imminently and moving as quickly as possible to initiate a registrational study for LX2006 by early 2026. We were also proud to share the promising, early data for LX2020 for the treatment of PKP2-associated arrhythmogenic cardiomyopathy and we look forward to sharing additional clinical updates later in 2025 for this program.” Business and Program Updates LX2006 for the Treatment of FA Cardiomyopathy: In April 2025, Lexeo announced positive interim data of LX2006 across both the Lexeo-sponsored SUNRISE-FA Phase 1/2 clinical trial (NCT05445323) and the Weill Cornell Medicine investigator-initiated Phase 1A trial (NCT05302271). Efficacy: Clinically meaningful improvements were observed across cardiac biomarkers and functional measures in the majority of participants across both studies. Participants with abnormal left ventricular mass index (LVMI) at baseline achieved 25% mean reduction in LVMI by 12 months or sooner, exceeding the 10% target reduction in LVMI by 12 months aligned with the U.S. Food and Drug Administration (FDA) for the planned registrational study.Protein Expression: Increases in cardiac frataxin expression were observed in all SUNRISE-FA participants at 3-months post treatment, with an average increase of 115% over baseline observed in the high-dose cohort.Safety: LX2006 continues to be generally well tolerated with no new treatment-related serious adverse events to report.Regulatory Plans: Lexeo expects final alignment with FDA on the LX2006 planned pivotal study protocol and statistical analysis plan in 2025. Lexeo previously aligned with FDA on co-primary endpoints for the study and measurement thresholds: greater than 10% reduction in LVMI as measured by cardiac MRI, and any increase from baseline cardiac frataxin expression as measured by liquid chromatography mass spectrometry (LCMS).Next Steps: In Q2 2025, Lexeo expects to begin enrollment in a prospective natural history study serving as a concurrent external control arm for the registrational study. The Company expects to initiate a registrational study by early 2026 with a potential efficacy readout in 2027. LX2020 for the Treatment of PKP2-ACM: In March 2025, Lexeo shared positive interim data of LX2020 from the low-dose cohort in the HEROIC-PKP2 Phase 1/2 clinical trial. Cohort 1 Interim Update: At 3-months post-treatment, cardiac biopsies from two participants in cohort 1 showed 71% and 115% increases, respectively, in PKP2 protein expression from baseline; the third cohort 1 participant elected not to undergo a post-treatment biopsy. The first participant evaluated 6-months post treatment experienced a 67% reduction in premature ventricular contractions (PVCs) from baseline.Safety: LX2020 has been generally well tolerated with no treatment-related serious adverse events to date across both dose cohorts.Next Steps: Currently enrolling cohort 3 of LX2020 HEROIC-PKP2 (n=4), with an interim clinical data update expected in the second half of 2025. Capital Redeployment and Cash Runway: In April 2025, Lexeo identified approximately $20 million in capital to redeploy towards the Company’s lead cardiac programs, LX2006 and LX2020. This capital was redeployed from preclinical and non-cardiac pipeline activities and included a limited reduction in force impacting approximately 15% of employees. The updated capital structure is expected to enable the Company to execute against key milestones for its clinical-stage pipeline, accelerate work to initiate a registrational study for LX2006 by early 2026, and maintain operational runway into 2027. First Quarter Financial Results Cash Position: As of March 31, 2025, cash, cash equivalents, and investments were $106.9 million, which Lexeo believes will be sufficient to fund operations into 2027.Research and Development Expenses: Research and Development expenses were $17.2 million for the three months ended March 31, 2025, compared to $15.7 million for the three months ended March 31, 2024.General and Administrative Expenses: General and Administrative expenses were $16.6 million for the three months ended March 31, 2025, compared to $7.5 million for the three months ended March 31, 2024.Net Loss: Net loss was $32.7 million or $0.99 per share (basic and diluted) for the three months ended March 31, 2025, compared to $21.7 million or $0.77 per share (basic and diluted) for the three months ended March 31, 2024. About Lexeo TherapeuticsLexeo Therapeutics is a New York City-based, clinical stage genetic medicine company dedicated to reshaping heart health by applying pioneering science to fundamentally change how cardiovascular diseases are treated. The Company is advancing a portfolio of therapeutic candidates that take aim at the underlying genetic causes of conditions, including LX2006 for the treatment of Friedreich ataxia (FA) cardiomyopathy, LX2020 for the treatment of plakophilin-2 (PKP2) arrhythmogenic cardiomyopathy, and others for devastating diseases with high unmet need. Cautionary Note Regarding Forward-Looking StatementsCertain statements in this press release may constitute “forward-looking statements” within the meaning of the federal securities laws, including, but not limited to, Lexeo’s expectations and plans regarding its current product candidates and programs and the timing for receipt and announcement of data from its clinical trials, the timing and likelihood of potential regulatory developments and approval, expectations regarding the time period over which Lexeo’s capital resources will be sufficient to fund its anticipated operations and estimates regarding Lexeo’s financial condition. Words such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “develop,” “plan” or the negative of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While Lexeo believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements. These forward-looking statements are based upon current information available to the company as well as certain estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Lexeo’s filings with the U.S. Securities and Exchange Commission (SEC)), many of which are beyond the company’s control and subject to change. Actual results could be materially different from those indicated by such forward-looking statements as a result of many factors, including but not limited to: risks and uncertainties related to global macroeconomic conditions and related volatility; expectations regarding the initiation, progress, and expected results of Lexeo’s preclinical studies, clinical trials and research and development programs; the unpredictable relationship between preclinical study results and clinical study results; delays in submission of regulatory filings or failure to receive regulatory approval; liquidity and capital resources; and other risks and uncertainties identified in Lexeo’s Annual Report on Form 10-K for the annual period ended December 31, 2024, filed with the SEC on March 24, 2025, and subsequent future filings Lexeo may make with the SEC. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Lexeo claims the protection of the Safe Harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements. Lexeo expressly disclaims any obligation to update or alter any statements whether as a result of new information, future events or otherwise, except as required by law. Media Response:Media@lexeotx.com Investor Response:Carlo Tanzi, Ph.D.ctanzi@kendallir.com Lexeo Therapeutics, Inc.Selected Financial Information(in thousands, except share and per share amounts) Statements of Operations Three Months Ended March 31, 2025 2024 (unaudited) (unaudited)Operating expenses Research and development$17,171 $15,742 General and administrative 16,634 7,549 Total operating expenses 33,805 23,291 Operating loss (33,805) (23,291)Other income and expense Other income (expense), net (4) (5)Interest expense (28) (37)Interest income 1,193 1,651 Amortization of premium on investments (12) – Total other income and expense 1,149 1,609 Loss from operations before income taxes (32,656) (21,682)Income taxes – – Net loss$(32,656) $(21,682)Net loss per common share, basic and diluted$(0.99) $(0.77)Weighted average number of shares outstanding used in computation of net loss per common share, basic and diluted 33,113,991 27,979,838 Balance Sheet Data March 31, December 31, 2025 2024 (unaudited) Cash, cash equivalents, and investments$106,866 $128,530 Total assets 125,690 146,942 Total liabilities 37,575 30,100 Total stockholders’ equity 88,115 116,842
VUNO’s AI-Powered Cardiac Arrest Risk Management System Earns CE MDR and UKCA Certifications
Early Regulatory Approval Accelerates Expansion Plans in Europe and the Middle East SEOUL, South Korea, May 12, 2025 /PRNewswire/ — VUNO, a leading South Korean medical AI company, announced today that its flagship AI-powered cardiac arrest risk management system VUNO…
Tampa General Hospital and USF Health Complete First TricValve® Procedure in Florida in FDA Early Feasibility Study
The novel treatment is in development to address critical cardiac patients with a life-threatening condition and represents a significant milestone for the academic medical system’s minimally invasive valve program. TAMPA, Fla., May 9, 2025 /PRNewswire/ — Interventional structural…