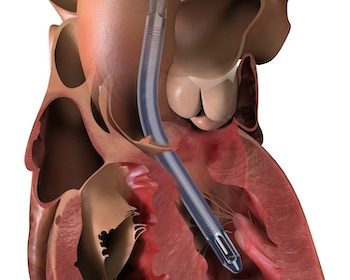
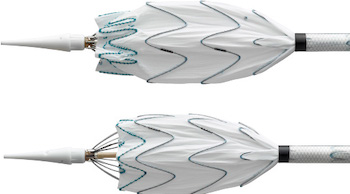
DANVERS, Mass.–(BUSINESS WIRE)–Three cardiac surgeons at Cleveland Clinic, Hackensack Meridian Health and Cedars-Sinai Medical Center are the first in the United States to implant Abiomed’s (NASDAQ: ABMD) newest heart pump, the Impella 5.5 with SmartAssist. Ed Soltesz, MD, Mark Anderson, MD, and Danny Ramzy, MD, have each successfully implanted multiple pumps during cardiac procedures at their hospitals. Mark […]
Other News
myTAIHEART Test Provides Evidence for Injury from Biopsy of Heart Transplant Recipients
MILWAUKEE, Wis.–(BUSINESS WIRE)–TAI Diagnostics, Inc., focused on developing innovative diagnostic tests for monitoring the health of transplanted organs, today announced the publication, “Effect of endomyocardial biopsy on levels of donor-specific cell-free DNA” in the October, 2019 issue of The Journal of Heart and Lung Transplantation. The clinical study presented in the paper was conducted jointly […]
Abiomed Announces Q2 FY 2020 Revenue of $205 Million and 29.4% Operating Margin
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart recovery and support technologies, today reported second quarter fiscal 2020 revenue of $205.0 million, an increase of 13% compared to revenue of $181.8 million for the same period of fiscal 2019. Operating income was $60.2 million, up 20%, compared […]
Teleflex Announces Quarterly Dividend
WAYNE, Pa., Oct. 31, 2019 (GLOBE NEWSWIRE) — Teleflex Incorporated (NYSE: TFX) announced today that its Board of Directors declared a quarterly cash dividend of thirty-four cents ($0.34) per share of common stock. The dividend is payable December 16, 2019 to shareholders of record at the close of business on […]
Merit Medical Reports Earnings for Third Quarter of 2019
Q3 2019 worldwide revenue of $243.0 million, up 9.6% as reported over Q3 2018 Q3 2019 core revenue on a comparable, constant currency basis* up 4.3% over Q3 2018 Q3 2019 GAAP loss per share was $(0.06), compared to GAAP EPS of $0.30 in Q3 2018 Q3 2019 non-GAAP EPS* […]
Endonovo Therapeutics Announces Reverse Stock Split
Los Angeles, CA, Oct. 31, 2019 (GLOBE NEWSWIRE) — Endonovo Therapeutics, Inc. (OTCQB: ENDV) (“Endonovo” or the “Company”) today announced that it had filed a preliminary information statement with respect to a proposed a reverse stock split of its common stock at a ratio of between 1 for 100 and […]
Medtronic Announces Shonin Approval and Launch of the Valiant Navion™ Thoracic Stent Graft System in Japan
Lower-Profile Thoracic Endovascular Aortic Repair (TEVAR) Device Continues to Broaden Global Treatable Patient Population with Thoracic Aortic Disease DUBLIN, Oct. 31, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced Shonin approval from the Ministry of Health, Labour and Welfare (MHLW) and the launch of the Valiant Navion™ thoracic stent graft system […]
Baylis Medical Introduces a 2-French Electrophysiology Catheter into North America
TORONTO, Oct. 31, 2019 /PRNewswire/ – Baylis Medical announced today the first North American use of its EPstar™ Fixed Electrophysiology Catheters. The EPstar catheters make available the smallest diagnostic catheter in the North American electrophysiology market, allowing physicians to reach previously inaccessible areas of the heart for procedure mapping. The EPstar […]
ANI Announces Plans to Launch Bretylium Tosylate Injection, USP 500mg/10ml (50mg/ml) for Ventricular Arrhythmias
BAUDETTE, Minn., Oct. 31, 2019 /PRNewswire/ — ANI Pharmaceuticals, Inc. (“ANI”) (Nasdaq: ANIP) today announced that its partner Pharmaceutics International Inc. (Pii) has received FDA approval of a Prior Approval Supplement for Bretylium Tosylate Injection, USP 500mg/10ml (50mg/ml). ANI plans to launch this currently unavailable drug in December, introducing this critical drug for the treatment of ventricular fibrillation and life-threatening […]
CryoLife Reports Third Quarter 2019 Financial Results
ATLANTA, Oct. 30, 2019 /PRNewswire/ — Third Quarter and Recent Business Highlights: Total revenues were $67.9 million in the third quarter of 2019, reflecting year over year growth of 5% and a 6% increase on a non-GAAP constant currency basis, both compared to the third quarter of 2018 On-X® revenues increased 12%, and 12% on […]