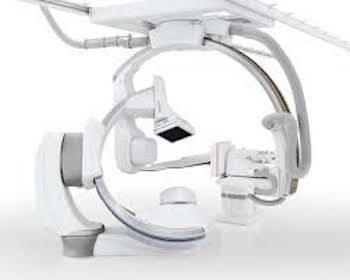
Program Provides Latest Interventional Technology While Reducing Cost and Installation Time TUSTIN, Calif.–(BUSINESS WIRE)–In the complex and fast-paced world of health care, customers face the challenge of staying up-to-date with the most advanced systems while also keeping costs down. Providers now have a solution: the Alphenix Encore Plus Program from Canon Medical Systems […]
Other News
Blood Flow Monitoring Device – FlowMet-R Gets FDA 510K Clearance
IRVINE, Calif., Oct. 2, 2019 /PRNewswire/ — Laser Associated Sciences (LAS), a medical device company based in Irvine, California, announced that its blood flow monitoring system, FlowMet-R, received its 510(k) clearance from the Food and Drug Administration (FDA). This clearance allows LAS to market and sell the FlowMet-R. The non-invasive portable device addresses a […]
AngioDynamics Acquires Eximo Medical, Ltd. and its Innovative 355nm Laser Atherectomy Technology
LATHAM, N.Y.–(BUSINESS WIRE)–AngioDynamics, Inc. (NASDAQ: ANGO), a leading provider of innovative, minimally invasive medical devices for vascular access, peripheral vascular disease, and oncology, today announced that it has acquired Eximo Medical, Ltd., an early commercial stage, medical device company, and its proprietary 355nm wavelength laser-technology platform for $46 million in […]
Milestone Pharmaceuticals Announces First Patient Enrolled in NODE-303 Open-label Safety Study of Etripamil in PSVT
MONTREAL and CHARLOTTE, N.C., Oct. 3, 2019 /PRNewswire/ — Milestone Pharmaceuticals Inc. (Nasdaq: MIST), a Phase 3 clinical-stage biopharmaceutical company dedicated to developing and commercializing etripamil for the treatment of cardiovascular indications, today announced that the first patient has been enrolled in the Phase 3 NODE-303 study. NODE-303 is the Company’s open-label, global safety study of etripamil, […]
Tenaya Therapeutics Closes $92 Million Series B Financing
SOUTH SAN FRANCISCO, Calif., Oct. 3, 2019 /PRNewswire/ — Tenaya Therapeutics, Inc., a company with a mission to discover, develop, and deliver curative therapies that target the underlying causes of heart disease, today announced the successful completion of a $92 million Series B financing. The financing round was led by Casdin Capital and included […]
Quantum Genomics Reports First Half 2019 Financial Results and Provides Corporate Update
Arterial hypertension: preparing for the pivotal Phase III study of firibastat in patients with resistant arterial hypertension Heart failure: the first patients were enrolled in June in QUORUM, a Phase IIb study of firibastat in heart failure PARIS and NEW YORK, Oct. 03, 2019 (GLOBE NEWSWIRE) — Quantum Genomics (Euronext Growth – FR0011648971 – ALQGC, OTCQX […]
Verve Therapeutics Appoints Cardiologist Andrew Bellinger, M.D., Ph.D., as Chief Scientific Officer
CAMBRIDGE, Mass.–(BUSINESS WIRE)–Verve Therapeutics, a next-generation cardiovascular company developing therapies that safely edit the adult human genome to permanently reduce a person’s risk of coronary artery disease, today announced the appointment of physician and scientist Andrew Bellinger, M.D., Ph.D., as the company’s chief scientific officer. Dr. Bellinger joins Verve from […]
University Hospitals Completes First Evolut™ PRO+ Case in the World
UH Harrington Heart & Vascular Institute continues reputation as global leader in TAVR CLEVELAND — Physicians at University Hospitals Cleveland Medical Center completed the first procedure in the world using Medtronic’s new Evolut™ PRO+ TAVR System. Guilherme Attizzani, MD, Director, Valve and Structural Heart Disease Center, and Cristian Baeza, […]
Third-party evaluation confirms safety profile of Philips Stellarex .035″ low-dose drug-coated balloon
Primary safety analysis of Stellarex drug-coated balloon (DCB) three-year data, comprising the largest published, pooled set of randomized controlled trial (RCT) data for a single paclitaxel-based device, shows no difference in mortality between patients treated with the Philips Stellarex DCB and those treated with percutaneous angioplasty, the current standard of […]
Endotronix Closes Expanded Series D Financing Round of $70 Million for Heart Failure Solution
LISLE, Ill, Oct. 1, 2019 /PRNewswire/ — Endotronix, Inc., a digital health, medtech company dedicated to advancing the treatment of heart failure (HF), today announced the expansion of its Series D financing round, bringing the total for the round to $70 million. The expansion syndicate for the LSP-led round includes new investment from an additional […]