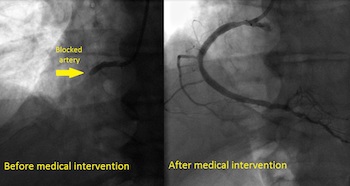
Growing Body of Evidence Supporting Use of the Tack Endovascular System® to Improve Balloon Angioplasty Outcomes for Patients Suffering from PAD WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced the positive one-year results of its Tack Optimized Balloon Angioplasty (TOBA) III clinical trial, successfully achieving […]
Other News
UH Portage Medical Center Achieves 6 Minute “Door-to-Balloon Time”
During a heart attack, cardiologists say “time is muscle.” The time between when you arrive at the hospital and when your blockage is opened matters. The American Heart Association recommends a “door-to-balloon time” (D2B) of no more than 90 minutes. University Hospitals Portage Medical Center in Ravenna, Ohio blew that […]
Medtronic Announces Early Feasibility Trial for Intrepid™ Transcatheter Mitral Valve Replacement System with Transfemoral Transseptal Approach
New Study Shows Momentum in Establishing a Less Invasive Approach to Treat Patients with Severe Mitral Valve Disease DUBLIN, Sept. 27, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced it has received U.S. Food and Drug Administration (FDA) approval to begin an early feasibility study (EFS) for its Intrepid™ transcatheter mitral […]
Boston Scientific Announces Positive Data from the EVOLVE Short DAPT study with the SYNERGY™ Bioabsorbable Polymer Stent
Study of abbreviated antiplatelet therapy for patients at high risk for bleeding after undergoing percutaneous coronary intervention SAN FRANCISCO and MARLBOROUGH, Mass., Sept. 26, 2019 /PRNewswire/ — Boston Scientific (NYSE: BSX) has announced primary endpoint results from the EVOLVE Short DAPT clinical trial, the first prospective study initiated in the U.S. to examine the safety […]
Resverlogix Announces US$12 Million Debenture Financing and Full Repayment of Loan
CALGARY, Alberta, Sept. 27, 2019 (GLOBE NEWSWIRE) — Resverlogix Corp. (“Resverlogix” or the “Company”) (TSX: RVX) today announces that it has issued a 10% secured convertible debenture in the principal amount of US$12 million (the “Debenture”) to a wholly-owned subsidiary of ORI Star Fund LP (“ORI” or the “Fund”). The […]
Resolute Onyx™ DES Meets Primary Endpoint in First-Ever Clinical Study Comparing Drug-Eluting Stents in High-Bleeding Risk (HBR) Patients with One-Month DAPT
DUBLIN and SAN FRANCISCO, Sept. 26, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) announced today late-breaking clinical data from the Onyx ONE Global Study, representing the first prospective, multi-center, randomized study evaluating clinical outcomes between two drug-eluting stents (DES) in nearly 2,000 high-bleeding risk (HBR) patients with one month of dual antiplatelet therapy […]
Access Vascular Secures New Financing to Accelerate Commercialization
BEDFORD, Mass., Sept. 26, 2019 /PRNewswire/ — Access Vascular, a medical device company developing the next generation of intravenous devices from novel biomaterials designed to minimize the risk of bloodstream infection, catheter thrombosis, and vein trauma, announced $6M in new funding from new and existing investors. With this new round, Access Vascular is poised to […]
BD Announces Leadership Succession Plan
FRANKLIN LAKES, N.J., Sept. 26, 2019 /PRNewswire/ — BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced that Vincent A. Forlenza, chairman and CEO, will retire as CEO on Jan. 28, 2020, following the company’s annual meeting of shareholders. Forlenza will continue on the BD board of directors, serving as […]
FDA Clears Modules of AI-Rad Companion Chest CT From Siemens Healthineers
MALVERN, Pa.–(BUSINESS WIRE)– The U.S. Food and Drug Administration (FDA) has cleared three modules of AI-Rad Companion Chest CT¹, an intelligent software assistant from Siemens Healthineers that brings artificial intelligence (AI) to computed tomography (CT). Representing the first intelligent assistant of the new AI-Rad Companion platform, AI-Rad Companion Chest CT […]
CorMatrix® Cardiovascular, Inc. completes enrollment of adult arm of its FDA early feasibility IDE study for the Cor® TRICUSPID ECM® valve for pediatric and adult patients
ATLANTA, Sept. 26, 2019 /PRNewswire/ — CorMatrix® Cardiovascular, Inc. www.cormatrix.com, a leading developer of regenerative cardiovascular medical devices, today announced it has completed enrollment of the adult arm of its FDA early feasibility IDE study for the Cor® TRICUSPID ECM® cardiac valve* for adults with endocarditis and for pediatric patients with congenital heart valve disease. The Cor® TRICUSPID ECM® valve […]