SANTA CLARA, Calif.–(BUSINESS WIRE)–Ancora Heart, Inc., a company developing a novel therapy to address heart failure, today announced results from an interim analysis of heart failure patients treated in the CorCinch FMR study, a U.S. early feasibility study evaluating the safety of the investigational AccuCinch® Ventricular Repair System designed for the […]
Other News
United Biologics, Inc. Appoints John Barnhill as Its President and General Manager
SANTA ANA, Calif.–(BUSINESS WIRE)–United Biologics, Inc., a global leader in silicone vasculature, today announced the appointment of John Barnhill as President and General Manager. As President and General Manager, Mr. Barnhill will guide the company’s commercial advancement and innovation strategies, as well as oversee all financial and operations infrastructure to […]
Abiomed Receives FDA PMA Approval for Impella 5.5 with SmartAssist, a Minimally Invasive, Forward Flow Heart Pump
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed’s (NASDAQ: ABMD) newest heart pump, the Impella 5.5 with SmartAssist, has received U.S. Food and Drug Administration (FDA) pre-market approval (PMA) for safety and efficacy in the therapy of cardiogenic shock for up to 14 days. Impella 5.5 with SmartAssist is: Minimally invasive, eliminating the need for a […]
OrbusNeich® Announces Japan Approval for COMBO® Plus Coronary Stent
HONG KONG, Sept. 25, 2019 /PRNewswire/ — OrbusNeich Medical K.K. of Tokyo Japan has announced that the Japan Ministry of Health, Labour, and Welfare (MHLW) has granted Shonin market approval for the COMBO Plus Coronary Stent. The COMBO Plus Coronary Stent is the first drug-eluting stent [DES] to combine the proprietary endothelial progenitor cell […]
Fitbit and FibriCheck Announce Partnership to Deliver CE-Marked Heart Health Detection App to Fitbit Smartwatch Users in Europe
Fitbit users in Belgium, the Netherlands, Ireland and the United Kingdom can use FibriCheck to help detect heart rhythm irregularities like Atrial Fibrillation SAN FRANCISCO–(BUSINESS WIRE)–Fitbit (NYSE:FIT) and FibriCheck, an innovative health screening and monitoring app, today announced a partnership enabling users in Belgium, the Netherlands, Ireland and the UK to […]
U.S. FDA Clears Abbott’s High Sensitivity Troponin-I Blood Test That Aids Doctors in Diagnosing Heart Attacks Faster and More Accurately
Newly cleared diagnostic test could help identify heart attacks several hours sooner than standard troponin tests and help improve diagnosis in women ABBOTT PARK, Ill., Sept. 25, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced that its ARCHITECT STAT High Sensitivity Troponin-I blood test has received clearance from the U.S. Food and Drug Administration […]
AI-Powered Digital Health Company Eko Closes $20 Million Series B to Continue the Fight Against Heart Disease
Led by ARTIS Ventures, Funding will Drive Further Research and Commercialization of Eko’s Machine Learning Platform San Francisco, CA, Sept. 25, 2019 (GLOBE NEWSWIRE) — Eko, a Silicon Valley-based digital health company building machine learning tools to fight heart disease, today announced it has secured $20 million in Series B funding. […]
Colibri Heart Valve Receives a United States Patent Directed to a Replacement Heart Valve that Includes a Dry Valve Sutured to a Stent Member
BROOMFIELD, CO, Sept. 24, 2019 (GLOBE NEWSWIRE) — Colibri Heart Valve LLC, a privately held emerging medical device company, today announced that U.S. Patent No. 10,390,945 (“the ‘945 Patent”) was issued on August 27, 2019 by the United States Patent & Trademark Office with a priority date extending back to July […]
Shockwave Study Demonstrates Strong Safety and Procedural Success of Coronary IVL in Europe
TCT Presentation of DISRUPT CAD II EU Post-Market Study Confirms Promissory DISRUPT CAD I Results Data Published Simultaneously in Circulation: Cardiovascular Interventions SANTA CLARA, Calif., Sept. 25, 2019 (GLOBE NEWSWIRE) — Shockwave Medical, Inc. (NASDAQ: SWAV), a pioneer in the development of Intravascular Lithotripsy (IVL) to treat complex calcified cardiovascular disease, unveiled […]
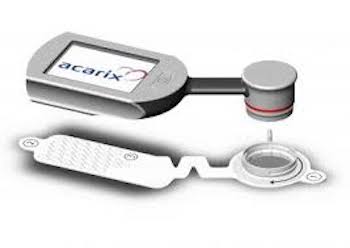
Acarix carries out a rights issue of approximately SEK 51.8 million to finance an increased market expansion
Malmö, 25 September 2019 Acarix carries out a rights issue of approximately SEK 51.8 million to finance an increased market expansion The board of directors of Acarix AB (“Acarix” or the “Company”) has today, pursuant to the authorization granted by the extra general meeting on 16 August 2019, resolved to […]