Atrial functional mitral regurgitation (aFMR) is associated with left atrial (LA) and mitral annular dilatation most often caused by atrial fibrillation or heart failure with preserved ejection fraction (HFpEF).
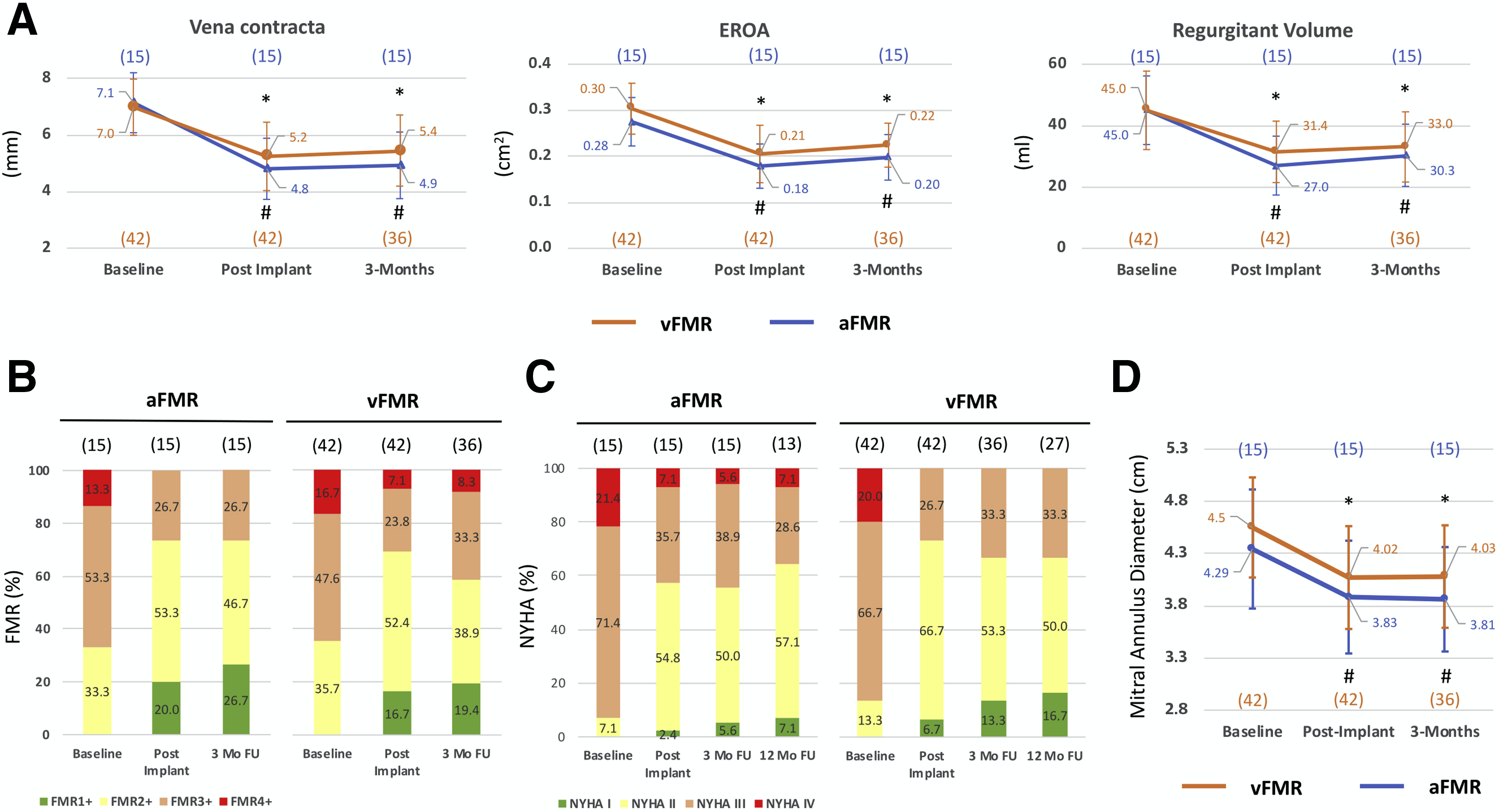
(A) Echocardiographic assessment, (B) functional mitral regurgitation classification, (C) New York Heart Association classification, and (D) mitral annulus diameter at baseline, post-implantation, 3 months, and for New York Heart Association classification also 12 months in atrial functional and ventricular secondary mitral regurgitation. Mean SD. *Versus vFMR baseline, p < 0.05. #Versus aFMR baseline, p < 0.05. aFMR ¼ atrial functional mitral regurgitation; EROA ¼ effective regurgitant orifice area; FU ¼ follow-up; NYHA ¼ New York Heart Association; vFMR ¼ ventricular secondary mitral regurgitation.
Characteristics of aFMR are normal left ventricular function, normal mitral leaflet motion, a more central regurgitation jet, and severe LA dilatation. In ventricular secondary mitral regurgitation (vFMR) systolic left ventricular dysfunction and restricted leaflet motion are pathogenetic. In contrast to vFMR little is known about the therapeutic options in aFMR (1). Mitral valve repair via the coronary sinus is 1 option for the treatment of vFMR. The CarillonMitral-Contour-System (Cardiac Dimensions, Kirkland, Washington) has demonstrated a reduction in vFMR and improved clinical symptoms (2,3). Currently no clinical data about Carillon device implantation in aFMR are available, so we aimed to analyze our first results of mitral annuloplasty in aFMR and compare them with the outcomes of patients with vFMR.
A written consent was obtained by every patient. Because we performed a retrospective analysis of our patient database no ethical approval according to local regulations was required.
Patients with symptomatic heart failure with reduced ejection fraction (left ventricular ejection fraction [LVEF] <50%) or HFpEF (LVEF >50%) suffering at least from moderate FMR were eligible for annuloplasty with the Carillon-Mitral-Contour-System (Cardiac Dimensions). aFMR was defined according to the following parameters: 1) at least moderate MR (FMR $2þ); 2) LVEF $50% 3) left ventricular enddiastolic diameter #55 mm; and 4) HFpEF (N-terminal pro–B-type natriuretic peptide $125 pg/ml, LA dilatation $34 ml/m2 , and echocardiographic signs of diastolic dysfunction [e.g., E/e’ $13]).
For statistical analysis all variables were tested for normal distribution (Kolmogorov-Smirnov test). The results are given as mean SD. Differences between groups were evaluated by 1-way analysis of variance with Scheffe post hoc testing. A p value < 0.05 was considered as statistically significant.
This retrospective study comprises 57 consecutive patients undergoing coronary sinus–based indirect mitral valve repair. Fifteen patients were classified to the aFMR and 42 to the vFMR group. In our patient cohort the mean age accounts for 76.9 1.6 years in aFMR and 75.6 1.4 years in vFMR. The majority of patients with aFMR were female (n ¼ 5; 66.7%), whereas patients with vFMR were predominantly male (n ¼ 32; 76.2%; p ¼ 0.002). LVEF was normal in aFMR (60.8 2.1%) and reduced in vFMR (30.1 1.4%; p < 0.001). Furthermore, left ventricular end-diastolic diameter was 47.0 1.3 mm in aFMR and elevated in vFMR (59.0 1.1 mm; p < 0.001). The LA was markedly dilated in aFMR in comparison with vFMR (aFMR, 69.3 5.2; vFMR, 51.3 3.2 ml/m2 ; p ¼ 0.004).
Echocardiographic assessment following mitral annuloplasty showed a comparable reduction in vena contracta, effective regurgitant orifice area, and regurgitant volume in aFMR and vFMR patients (Figure 1A). Furthermore, improved mitral regurgitation classification post-implantation and at 3-months follow-up was found in aFMR and vFMR (Figure 1B). After 3 months the number of patients with New York Heart Association functional class III decreased from 71.4% to 38.9% in aFMR and from 66.7% to 33.3% in vFMR, which remained stable after 12 months (Figure 1C). In both aFMR and vFMR, mitral annulus diameter was reduced to the same extent at 3-months follow-up (Figure 1D).
We analyzed for the first time the results of Carillon device implantation in aFMR compared with vFMR. Both groups showed a similar reduction of echocardiographic parameters and New York Heart Association functional class post-implantation. Other techniques, such as edge-to-edge repair or Cardioband, could also be potential effective therapies of aFMR. To date no prospective clinical trials have been published using these therapeutic options in aFMR. A reduced all-cause mortality following MitraClip implantation encourages to investigate this technique in aFMR (4). Of note, because of different therapeutic effects our results are not transferable to other mitral valve interventions. Therefore, future clinical trials of transcatheter mitral valve repair addressing aFMR are warranted.
This study is a retrospective, single-center study with a limited number of patients. The results should be regarded as hypothesis generating. In conclusion, indirect mitral valve annuloplasty using the Carillon device is feasible in HFpEF patients with aFMR.
Dennis Rottländer, MD
Miriel Gödde, MD
Hubertus Degen, MD
Alev Ögütcü, MD
*Michael Haude, MD, PhD
*Rheinland Klinikum Neuss–Medical Clinic I
Preussenstrasse 84
41464 Neuss
Germany
E-mail: mhaude@lukasneuss.de
https://doi.org/10.1016/j.jcin.2020.09.019
2020 by the American College of Cardiology Foundation. Published by Elsevier.
Dr. Degen is a consultant for Biotronik and Cardiac dimensions. Dr. Haude received institutional grants/research supports from Abbott and Biotronik; and received honoraria or consultation fees from Biotronik, Cardiac Dimensions, OrbusNeich, and Philips. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
1. Deferm S, Bertrand PB, Verbrugge FH, et al. Atrial functional mitral regurgitation: JACC Review Topic of the Week. J Am Coll Cardiol 2019;73:2465–76.
2. Witte KK, Lipiecki J, Siminiak T, et al. The REDUCE FMR trial: a randomized sham-controlled study of percutaneous mitral annuloplasty in functional mitral regurgitation. J Am Coll Cardiol HF 2019;7:945–55.
3. Lipiecki J, Siminiak T, Sievert H, et al. Coronary sinus-based percutaneous annuloplasty as treatment for functional mitral regurgitation: the TITAN II trial. Open Heart 2016;3:e000411.
4. Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitralvalve repair in patients with heart failure. N Engl J Med 2018;379: 2307–18.






