AMSTERDAM, June 27, 2017 /PRNewswire/ — Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market IntelliSpace Portal 9.0 and a range of innovative applications for Radiology in the U.S. The latest release of Philips’ clinical informatics platform for advanced visual analysis and quantification of medical images now offers enhanced additional applications for Longitudinal Brain Imaging and Multi-Modality Tumor Tracking, as well as optimized Lung Nodule Assessment.
IntelliSpace Portal 9.0 has been available outside of the U.S. since November 2016 and will be available in the world’s largest healthcare market as of this month.
Quantify and quickly diagnose conditions
The new innovations that are now cleared add to more than 70 applications that IntelliSpace Portal 9.0 offers on a single platform, spanning clinical domains within radiology, including neurology, oncology, and cardiology. The platform gives clinicians a comprehensive overview of each patient, helping them to quantify and quickly diagnose conditions using multimodality clinical applications that are optimized for patient evaluation over longer periods of time.
The number of people diagnosed with cancer and chronic diseases such as Cardiovascular Disease, COPD and Dementia continues to rise. Timely diagnosis and early intervention can improve patient outcomes. These conditions also require longer term treatment and continued follow-up to monitor a patient’s response to a chosen therapy over time and define the next steps in the treatment process.
“Analytics applications optimized for clinical decision support and longitudinal and quantified patient tracking are becoming increasingly important to radiologists,” said Mark van Buchem, professor of neuroradiology at the Leiden University Medical Center, one of the development partners of IntelliSpace Portal 9.0. “They can help visualize and quantify very subtle manifestations of disease and differences over time that may not be seen with the naked eye. IntelliSpace Portal 9.0 integrates into our existing workflow and adds greatly to our patient care.”
“IntelliSpace Portal shows how we continuously innovate to help clinicians achieve first-time-right diagnoses, track disease development, and improve patient care,” said Yair Briman, Business Leader Healthcare Informatics at Philips. “Together with clinical partners we develop solutions that help add depth and insight to a physician’s diagnosis. Our newest clinical informatics innovations can now be used to help streamline care for more and even the most complex patients.”
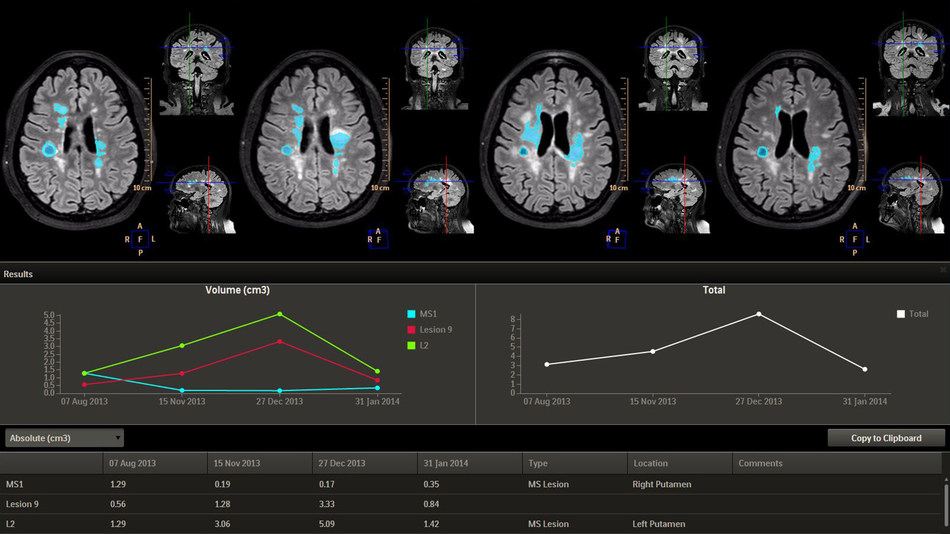
Evaluation of brain disorders over time
The newest clinical advance on the Intellispace Portal now cleared for U.S. distribution is its Longitudinal Brain Imaging (LoBI) – an application to analyze brain images to support the evaluation of neurological disorders over time. LoBI helps clinicians monitor disease progression in patients with neurodegenerative disorders such as stroke, Alzheimer’s disease, multiple sclerosis (MS).
Proprietary qEASL technology for oncology
The qEASL[1] capability within Multi-Modality Tumor Tracking offers a new method for enhanced measurement of tumor volume based on MRI and CT scans. This is a functionality aims to improve the current standard for cancer treatment follow-ups giving a visual indication of how cells respond to therapy. Also now available in the U.S. is Lung Nodule Assessment, which offers a diagnostic patient imaging tool that provides quantitative and characterization information about nodules in the lung in a single CT study, or over time during the course of multiple thoracic studies.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people’s health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips’ health technology portfolio generated 2016 sales of EUR 17.4 billion and employs approximately 70,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
[1] quantitative European Association for the Study of the Liver
For further information, please contact:
Kathy O’Reilly
Philips Group Press Office
Tel.: +1 978-659-2638
E-mail: Kathy.Oreilly@philips.com
Twitter: @kathyoreilly
Joost Maltha
Philips Group Press Office
Tel.: +31 610-55-8116
E-mail: Joost.Maltha@philips.com
Twitter: @JoostMaltha
SOURCE Royal Philips