First and Only Dual Monorail Microcatheter System Purpose-Built for Complex Coronary and Peripheral Interventions SAN JOSE, Calif., Oct. 25, 2024 /PRNewswire/ — Vantis Vascular, Inc., a pioneering medical technology company founded by physicians with a passion to revolutionize vascular…
Tag: 510K
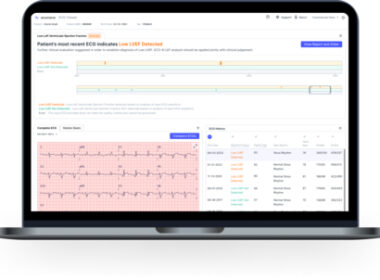
Anumana Receives U.S. FDA 510(k) Clearance for ECG-AI Algorithm to Detect Low Ejection Fraction
CAMBRIDGE, Mass.–(BUSINESS WIRE)–Anumana, Inc., a leading AI-driven health technology and nference portfolio company working in collaboration with Mayo Clinic, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for ECG-AI LEF, a breakthrough artificial intelligence (AI)-powered medical device to detect low ejection fraction (LEF) in patients at risk of heart failure. “Anumana’s […]
EndoVantage Secures 510(k) Clearance from FDA for SurgicalPreviewTM
SCOTTSDALE, AZ – November 8, 2017 – EndoVantage, LLC, a pioneer in cloud-based medical simulation technology, today announced its receipt of 510(k) clearance from the U.S. Food and Drug Administration for SurgicalPreviewTM, its preoperative planning system for the endovascular treatment of cerebral aneurysms. A cerebral aneurysm is a weak spot […]
SurModics (SRDX) Nabs Global Approvals of .014″ Low-Profile PTA Balloon Dilation Catheter
EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–Surmodics, Inc. (NASDAQ: SRDX), a leading provider of medical device and in vitro diagnostic technologies to the healthcare industry, announced it has received U.S. Food and Drug Administration (FDA) 510(k) and CE Mark clearance for its .014” low-profile percutaneous transluminal angioplasty (PTA) balloon dilation catheter, designed for […]
AUM CARDIOVASCULAR RECEIVES FDA CLEARANCE FOR REVOLUTIONARY DIAGNOSTIC HEART DEVICE
NORTHFIELD, MINNESOTA— AUM Cardiovascular announced that it has received clearance from the Food and Drug Administration for CADence,™ a non-invasive acoustic and ECG device designed to help physicians detect physiological and pathological heart murmurs. The reusable, non-invasive, radiation-free handheld device, which is now available in the United States, records sounds […]