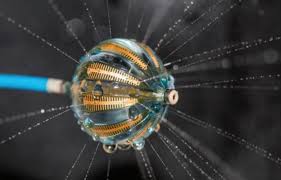
MINNEAPOLIS–(BUSINESS WIRE)– Imricor Medical Systems announced today that the results from the clinical study to evaluate Imricor’s Vision-MRTM Ablation Catheter for the treatment of atrial flutter under real-time MRI guidance will be presented this week at the Heart Rhythm Society (HRS) conference in Boston. Philipp Sommer MD will present the study results on […]
Tag: A-Fib
BIOSENSE WEBSTER, INC. ANNOUNCES FIRST PATIENT TREATED IN STUDY OF MULTI-ELECTRODE RADIOFREQUENCY BALLOON CATHETER
Multicenter Study to Evaluate Balloon Ablation Catheter for Safety and Effectiveness in Achieving Pulmonary Vein Isolation in Treatment of Paroxysmal Atrial Fibrillation IRVINE, CA, MARCH 15 – Johnson & Johnson Medical Devices Companies* announced today that Biosense Webster, Inc., a worldwide leader in the diagnosis and treatment of heart arrhythmias, […]
PATIENT ENROLLMENT COMPLETED IN U.S. IDE STUDY OF THERMOCOOL SMARTTOUCH® SF CATHETER FOR TREATMENT OF PERSISTENT ATRIAL FIBRILLATION
IRVINE, CA, — March 13, 2018 — Johnson & Johnson Medical Devices Companies* announced today that Biosense Webster, Inc., a worldwide leader in the diagnosis and treatment of heart arrhythmias, has completed patient enrollment in its U.S. Investigational Device Exemption (IDE) study of the THERMOCOOL SMARTTOUCH® SF Catheter. The prospective, […]
SentreHEART Announces Participation at Transcatheter Cardiovascular Therapeutics 2017
REDWOOD CITY, Calif.–(BUSINESS WIRE)–SentreHEART, Inc., the manufacturer of the LARIAT® Suture Delivery Device (LARIAT) will be participating in the Transcatheter Cardiovasular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, in Denver, CO October 29 – November 2, 2017. The symposium’s agenda will include several sessions dedicated to […]
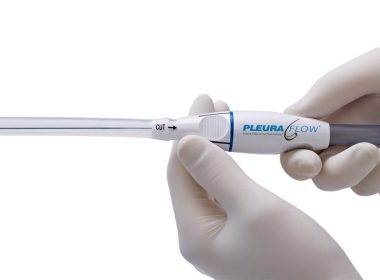
Newly Published Peer Reviewed Study Reveals Benefits Of ClearFlow’s PleuraFlow ACT System
ANAHEIM, Calif.–(BUSINESS WIRE)–ClearFlow Inc., a medical device company based in Anaheim, California, has announced the publication of significantly positive results in a study evaluating the company’s PleuraFlow® Active Clearance Technology® System. Data from a peer-reviewed clinical study indicating a marked reduction in Postoperative Atrial Fibrillation (POAF) among patients with active clearance of chest […]
Boehringer Ingelheim Release: Pradaxa (Dabigatran Etexilate) Dual Therapy Showed Lower Rates Of Major Bleeding Versus Triple Therapy With Warfarin In Atrial Fibrillation Patients Undergoing Stent Placement
RE-DUAL PCI™ showed large reductions in the incidence of bleeding complications if Pradaxa® dual therapy was used instead of warfarin triple therapy. Both Pradaxa® doses tested in RE-DUAL PCI™ have been approved for stroke prevention in atrial fibrillation. Data were presented as a late-breaker at the ESC Congress 20171 and published in the […]
Cardiome Provides U.S. Regulatory Update For BRINAVESS
VANCOUVER, Aug. 21, 2017 /PRNewswire/ – Cardiome Pharma Corp. (NASDAQ: CRME/ TSX:COM) today announced that it has received a response from the U.S. Food and Drug Administration (FDA) regarding the regulatory path for BRINAVESS® (vernakalant hydrochloride, IV), the Company’s antiarrhythmic drug for the rapid conversion of recent onset atrial fibrillation (AF). In its […]
CardioFocus® Receives Approval For HeartLight® Endoscopic Ablation System From The Japanese Ministry Of Health, Labour And Welfare
MARLBOROUGH, Mass., July 31, 2017 /PRNewswire/ — CardioFocus, Inc. today announced that the Japanese Ministry of Health, Labour and Welfare has approved the HeartLight Endoscopic Ablation System for the treatment of paroxysmal atrial fibrillation (AF) in Japan. The HeartLight System is a visually guided laser balloon technology for controlled and consistent pulmonary vein isolation (PVI) […]
CardioFocus Announces Initiation Of Three Major Studies Featuring The HeartLight System For The Treatment Of Atrial Fibrillation
MARLBOROUGH, Mass., June 29, 2017/PRNewswire/ — CardioFocus, Inc., a medical device innovator and manufacturer dedicated to advancing ablation treatment for cardiac disorders such as atrial fibrillation (AF), today announced that its HeartLight Endoscopic Ablation System is being featured in three new major clinical studies currently underway. Collectively, up to 1,000 AF patients will be […]
Texas Cardiac Arrhythmia Institute at St. David’s Medical Center First in Texas and Among First in U.S. to Use FlexAbility™ Ablation Catheter, Sensor Enabled™
AUSTIN, Texas, March 21, 2017 /PRNewswire/ — On March 1, 2017, the Texas Cardiac Arrhythmia Institute (TCAI) at St. David’s Medical Center was the first in Texas and among the first facilities in the U.S. to use the FlexAbility™ Ablation Catheter, Sensor Enabled™, designed to improve versatility and precision during […]