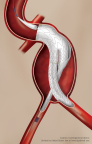
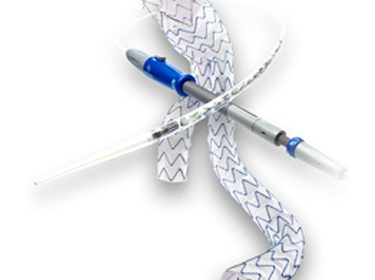
NEW YORK, Jan. 9, 2026 /PRNewswire/ — Major Medical Devices, Inc. (http://www.majormedicaldevices.com) (MMD), an emerging innovator in vascular intervention technologies with a highly differentiated, potentially quick to market device in a $3 billion market, announces strategic funding…
Tag: AAA
Nectero Medical Receives FDA Fast Track Designation for Nectero EAST® System
TEMPE, Ariz.–(BUSINESS WIRE)–Nectero Medical, a clinical-stage biotechnology company pioneering novel therapies to treat aneurysmal disease, today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for the Nectero Endovascular Aneurysm Stabilization Treatment (Nectero EAST®) System to treat patients with infrarenal AAAs, maximum diameter 3.5 – […]
Nectero Medical Receives FDA Clearance of IND Application to Initiate Phase II/III Clinical Trial of Nectero EAST® System for Treatment of Small- to Mid-Sized Abdominal Aortic Aneurysms
TEMPE, Ariz., July 24, 2023 /PRNewswire/ — Nectero Medical, a clinical-stage biotechnology company pioneering novel therapies to treat aneurysmal disease and improve patients’ lives, today announced that the U.S. Food and Drug Administration (FDA) granted Investigational New Drug (IND) clearance for the company to initiate a prospective, multi-center, randomized clinical trial (the […]
Viz.ai is First to Receive FDA 510(k) Clearance for AI Algorithm for Abdominal Aortic Aneurysm
New artificial intelligence algorithm flags and triages suspected abdominal aortic aneurysms SAN FRANCISCO – March 21, 2023- Viz.ai, the leader in AI-powered disease detection and intelligent care coordination, today announced it has received U.S. Food and Drug Administration (FDA) clearance for its algorithm intended to detect suspected abdominal aortic aneurysm […]
Viz.ai Launches AI-Powered Viz™ Vascular Suite
End-to-end platform for vascular care teams to accelerate the detection and treatment of suspected vascular disease SAN FRANCISCO–(BUSINESS WIRE)–Viz.ai, the leader in AI-powered disease detection and intelligent care coordination, today announced the launch of VizTM Vascular Suite, AI-powered software enabling vascular care teams to automatically detect and triage care for suspected […]
First Patient Enrolled in Investigational Study of the GORE EXCLUDER Conformable AAA Endoprosthesis with ACTIVE CONTROL System
FLAGSTAFF, Ariz.–(BUSINESS WIRE)– W. L. Gore & Associates, Inc.(Gore) today announced the first implant of the GORE® EXCLUDER® Conformable AAA Endoprosthesis in the United States. The successful procedure took place on December 19, 2017 at Maimonides Medical Center in New York by Robert Rhee, MD, Chief of Vascular and Endovascular Surgery, and National […]
CryoLife Announces Definitive Agreement to Acquire JOTEC
ATLANTA, Oct. 10, 2017 /PRNewswire/ — CryoLife, Inc. (“CryoLife”; NYSE: CRY), a leading medical device and tissue processing company focused on cardiac and vascular surgery, announced today that it has entered into a definitive agreement to acquire JOTEC AG (“JOTEC”). JOTEC is a German-based, privately-held developer of technologically differentiated endovascular stent grafts, […]
Medtronic (MDT)’s Stent System Wins FDA OK to Treat Short Neck Anatomies When Used With Heli-FX EndoAnchor System
DUBLIN – October 9, 2017 – Medtronic plc (NYSE: MDT) today announced that it has received U.S. Food and Drug Administration (FDA) approval for the Endurant(TM) II/IIs stent graft system to treat abdominal aortic aneurysm (AAA) patients with neck lengths down to 4mm and <=60° infra-renal angulation when used in combination […]
Endologix Receives IDE Approval For The EVAS2 Confirmatory Clinical Study To Evaluate The Nellix Endovascular Aneurysm Sealing System
IRVINE, Calif.–(BUSINESS WIRE)–Endologix, Inc. (Nasdaq:ELGX), a developer and marketer of innovative treatments for aortic disorders, announced today that it has received Investigational Device Exemption (“IDE”) approval from the United States Food and Drug Administration (“FDA”) to commence a confirmatory clinical study to evaluate the safety and effectiveness of the Nellix […]
Celebrating The 20th Anniversary Of The W. L. Gore & Associates EXCLUDER Device
FLAGSTAFF, Ariz.–(BUSINESS WIRE)–W. L. Gore & Associates, Inc. (Gore) today announced the 20th anniversary of the first implant of the GORE® EXCLUDER® AAA Endoprosthesis, the most-studied** endovascular aneurysm repair (EVAR) device on the market, with more than 300,000 patients treated* worldwide. The GORE EXCLUDER Device provides physicians with a proven and durable option to […]