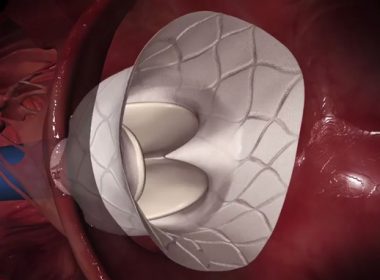
SAN DIEGO, Sept. 23, 2018 /PRNewswire/ — Abbott (NYSE :ABT ) today announced positive clinical study results from a randomized controlled trial comparing treatment with the MitraClip® device to guideline-directed medical therapy in select patients with secondary (or functional) mitral regurgitation, or a leaky heart valve, as a result of advanced heart failure. The landmark […]
Tag: Abbott
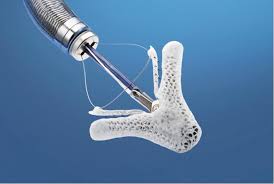
Abbott Begins U.S. Pivotal Trial for the Tendyne Mitral Valve Replacement System to Treat Patients with Heart Valve Disease
ABBOTT PARK, Ill., July 26, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced it has initiated a pivotal clinical study in the U.S. of its Tendyne Transcatheter Mitral Valve Replacement (TMVR) system for the treatment of mitral regurgitation. The trial will evaluate the safety and efficacy of the treatment in patients suffering from mitral […]
Abbott Reports Second-Quarter 2018 Results
ABBOTT PARK, Ill., July 18, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced financial results for the second quarter ended June 30, 2018. Second-quarter worldwide sales of $7.8 billion increased 17.0 percent on a reported basis and 8.0 percent on an organic* basis. Reported diluted EPS from continuing operations under GAAP was $0.40 in the second quarter. Adjusted diluted EPS […]
Abbott Receives FDA Approval for Next-Generation MitraClip® Device to Treat People with Leaky Heart Valves
ABBOTT PARK, Ill., July 12, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced it received approval from the U.S. Food and Drug Administration (FDA) for a next-generation version of its leading MitraClip® heart valve repair device used to repair a leaky mitral valve without open-heart surgery. The transcatheter clip-based therapy, now on a third generation […]
Abbott’s Investigational Tendyne™ Device for Mitral Valve Replacement Demonstrates Positive Outcomes at 30 Days in Global Study
PARIS, May 23, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced favorable outcomes from the first 100 patients treated in a global study of its Tendyne Transcatheter Mitral Valve Replacement (TMVR) system, the first and only mitral replacement valve that is repositionable and fully retrievable to allow for more precise implantation, helping improve […]
Newest Generation of Leading Heart Stent is Now Approved in the U.S. for People with Coronary Artery Disease
ABBOTT PARK, Ill., May 23, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced it received approval from the U.S. Food and Drug Administration (FDA) for XIENCE SierraTM, the newest generation of the company’s gold-standard XIENCE everolimus-eluting coronary stent system. XIENCE stents are among the world’s most-used and studied stents and have an exceptional safety […]
Five-Year Study Data Confirm Positive Outcomes for Patients When Abbott Diagnostic Tool Was Used to Guide Heart Stenting Decisions
PARIS, May 22, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced five-year results from the FAME 2 study, which showed that patients had fewer major adverse cardiac events (MACE) when they received a heart stent guided by Abbott’s fractional flow reserve (FFR) diagnostic tool in combination with medical therapy compared to patients […]
Abbott Expands Cardiac Arrhythmias Portfolio with FDA Clearance of Advanced Mapping Catheter
ABBOTT PARK, Ill., May 3, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced U.S. Food and Drug Administration (FDA) clearance of the Advisor™ HD Grid Mapping Catheter, Sensor Enabled™. Advisor HD Grid employs a new design that allows physicians to see things differently, capturing and analyzing data in a novel manner to create […]
Abbott’s XIENCE Sierra™ Heart Stent Receives National Reimbursement in Japan to Treat People with Coronary Artery Disease
ABBOTT PARK, Ill., May 2, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced that Japan’s Ministry of Health Labour and Welfare (MHLW) granted national reimbursement for XIENCE Sierra™, the newest generation of the company’s gold-standard XIENCE everolimus-eluting coronary stent. XIENCE Sierra improves upon previous versions of XIENCE with an enhanced stent design, a new delivery […]
Abbott Reports First-Quarter 2018 Results
ABBOTT PARK, Ill., April 18, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced financial results for the first quarter ended March 31, 2018. First-quarter worldwide sales of $7.4 billion increased 16.7 percent on a reported basis and 6.9 percent on an organic* basis. Reported diluted EPS from continuing operations under GAAP was $0.23 in the first quarter. Adjusted diluted EPS from […]