ABBOTT PARK, Ill., April 11, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced the initiation of a clinical trial evaluating long-term outcomes of patients who undergo stent implantation guided by high-resolution light-based imaging technology—called optical coherence tomography (OCT)—compared to a common X-ray-guided technique called angiography. The trial (ILUMIEN IV) is the first large-scale randomized global […]
Tag: Abbott
Abbott Initiates Trial to Evaluate Improved Survival And Outcomes with the CardioMEMS Monitor
ABBOTT PARK, Ill., March 29, 2018 /PRNewswire/ — Abbott (NYSE: ABT) announced today the company has initiated the landmark GUIDE-HF clinical trial using the CardioMEMS™ HF System. The GUIDE-HF trial will study whether the CardioMEMS device can improve survival and quality of life for people living with New York Heart Association (NYHA) Class II – IV heart failure. The […]
Abbott Hosts Conference Call for First-Quarter Earnings
ABBOTT PARK, Ill., March 21, 2018 /PRNewswire/ — Abbott (NYSE: ABT) will announce its first-quarter 2018 financial results on Wednesday, April 18, 2018, before the market opens. The announcement will be followed by a live webcast of the earnings conference call at 8 a.m. Central time (9 a.m.Eastern), and will be accessible through Abbott’s Investor Relations website […]
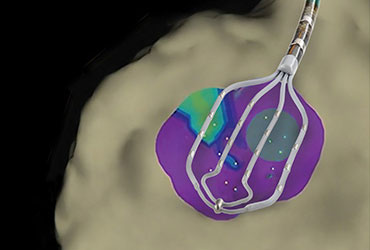
Abbott’s MitraClip Therapy Receives National Reimbursement in Japan to Treat Patients with Mitral Regurgitation
ABBOTT PARK, Ill., March 19, 2018 /PRNewswire/ — Abbott (NYSE: ABT) today announced that the Ministry of Health Labour and Welfare (MHLW) in Japan granted national reimbursement for the company’s MitraClip therapy to treat people with mitral regurgitation, a serious, progressive heart disease in which the mitral valve does not close properly, […]
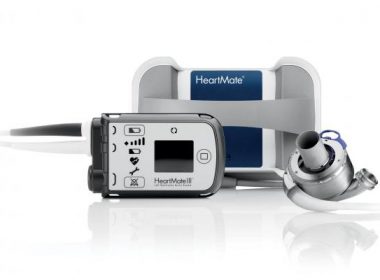
New Long-Term Data Show Improved Survival and Lower Rates of Stroke and Pump Thrombosis for Abbott’s HeartMate 3 Heart Pump
ORLANDO, Fla., March 11, 2018 /PRNewswire/ — Abbott (NYSE: ABT) announced new late-breaking clinical trial data from the MOMENTUM 3 clinical study, the largest left ventricular assist device (LVAD) trial in the world to evaluate patients in need of both short-term and long-term support in a single study. The data were published online […]
FDA Approves the World’s Smallest Mechanical Heart Valve for Pediatric Patients with Heart Defects
ABBOTT PARK, Ill., March 6, 2018 /PRNewswire/ — Abbott today announced the U.S. Food and Drug Administration (FDA) approved the Masters HP™ 15mm rotatable mechanical heart valve, the world’s smallest mechanical heart valve, that will allow doctors to treat babies and toddlers in need of a mitral or aortic valve replacement. Until […]
Abbott and Surmodics Announce Agreement for Next-Generation Drug-Coated Balloon
ABBOTT PARK, Ill. and EDEN PRAIRIE, Minn., Feb. 27, 2018 /PRNewswire/ — Abbott (NYSE: ABT) and Surmodics (NASDAQ: SRDX) today announced that the companies have entered into an agreement whereby Abbott will have exclusive worldwide commercialization rights for Surmodics’ SurVeil® drug-coated balloon to treat the superficial femoral artery, which is currently being evaluated in a U.S. pivotal […]
Abbott announces European launch of Advisor HD Grid Mapping Catheter, Sensor Enabled
ABBOTT PARK, Ill., Jan. 11, 2018 – Abbott today announced CE Mark approval for the company’s new Advisor™ HD Grid Mapping Catheter, Sensor Enabled™, a product designed to advance cardiac mapping during cardiac ablation to treat patients with complex cardiac arrhythmias. With the European launch of this latest addition to […]
Abbott Raising Quarterly Dividend for 46th Straight Year
ABBOTT PARK, Ill., Dec. 15, 2017 /PRNewswire/ — The board of directors of Abbott (NYSE: ABT) today increased the company’s quarterly common dividend to 28 cents per share from 26.5 cents per share. This marks the 376th consecutive quarterly dividend to be paid by Abbott since 1924. The cash dividend is payable Feb. 15, 2018, to shareholders of record at […]
ABBOTT’S MITRACLIP APPROVED AS FIRST TRANSCATHETER MITRAL VALVE REPAIR DEVICE IN JAPAN
ABBOTT PARK, Ill., Nov. 6, 2017 /PRNewswire/ — Abbott (NYSE: ABT) today announced that Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved the company’s MitraClip device for treatment of people with mitral regurgitation (MR), a serious, progressive heart disease in which the mitral valve does not close properly, allowing blood to flow backward […]