DENVER, Oct. 30, 2017 /PRNewswire/ — Abbott (NYSE: ABT) today announced that patients who underwent minimally-invasive implantation with a XIENCE coronary stent for left-main coronary artery disease had the same long-lasting health outcomes at three years but felt better more quickly than patients who underwent open-heart surgery. The data were presented during […]
Tag: Abbott
Abbott Launches the First and Only Smartphone Compatible Insertable Cardiac Monitor in the U.S.
ABBOTT PARK, Ill., Oct. 23, 2017 /PRNewswire/ — Abbott (NYSE: ABT) has secured U.S. Food and Drug Administration (FDA) clearance for the Confirm Rx™ Insertable Cardiac Monitor (ICM), the world’s first and only smartphone compatible ICM designed to help physicians remotely identify cardiac arrhythmias. With FDA clearance Abbott can now provide U.S. patients a new […]
FDA OKs Abbott (ABT)’ MRI-Compatibility for the Company’s Ellipse ICD
ABBOTT PARK, Ill., Sept. 22, 2017 /PRNewswire/ — Abbott (NYSE: ABT) today announced U.S. Food and Drug Administration (FDA) approval for magnetic resonance (MR)-conditional labeling for one of Abbott’s most widely-used implantable cardioverter defibrillators (ICD) and associated high voltage leads. The approval of MR-conditional labeling for the Ellipse ICD with the Tendril MRI […]
Abbott is stopping the sale of Absorb first generation BVS in all markets
Time to throw in the towel for Abbott on this generation of their Absorb BVS because of slow sales. However, there could still be a platform for this technology… Abbott halts global sales of 1st-gen Absorb stents SEPTEMBER 8, 2017 BY FINK DENSFORD , MassDevice Abbott (NYSE:ABT) said today it will halt global sales […]
ABBOTT ISSUES NEW UPDATES FOR IMPLANTED CARDIAC DEVICES
ABBOTT PARK, Ill., Aug. 29, 2017 – Today, Abbott notified physicians of updates to its implantable pacemakers and defibrillators as part of its ongoing commitment to continuously improve patient care. The new device updates include a Battery Performance Alert for our implantable cardioverter defibrillators (ICDs) that provides physicians with earlier […]
Abbott (ABT) Introduces Heartmate 3 Left Ventricular Assist System – The Latest Milestone In Therapy For Advanced Heart Failure Patients
ABBOTT PARK, Ill., Aug. 28, 2017 /PRNewswire/ — Abbott (NYSE: ABT) announced today it has received U.S. Food and Drug Administration (FDA) approval for its Full MagLev HeartMate 3 Left Ventricular Assist System (also known as an LVAD). The HeartMate 3 system provides a new option for physicians managing advanced heart failure patients in need […]
Abbott (ABT) Declares 374th Consecutive Quarterly Dividend
ABBOTT PARK, Ill., June 9, 2017 /PRNewswire/ — The board of directors of Abbott (NYSE: ABT) today declared a quarterly common dividend of 26.5 cents per share. This marks the 374th consecutive quarterly dividend to be paid by Abbott since 1924. The cash dividend is payable Aug. 15, 2017, to […]
Abbott (ABT) Announces CE Mark And First Use Of The World’s First Smartphone Compatible Insertable Cardiac Monitor
ABBOTT PARK, Ill., May 8, 2017 /PRNewswire/ — Abbott (NYSE: ABT) today announced CE Mark and first use of the new Confirm Rx Insertable Cardiac Monitor (ICM), the world’s first smartphone compatible ICM that will help physicians identify difficult to detect cardiac arrhythmias, including atrial fibrillation (AF), to help guide therapy. […]
Lessons We Can Learn From the Abbott BVS Data
By Ken Dropkiewski The bioresorbable stent was approved for use by the FDA in July of 2016. The Absorb GT1 Bioresorbable Vascular Scaffold System, manufactured by Abbott, was all set to solve a litany of the problems inherent with conventional cardiac and vascular stents. Conventional stents, made of flexible metal […]
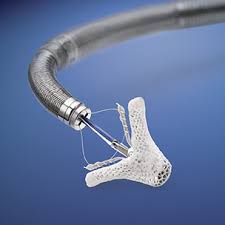
Late-Breaking Data on Abbott’s MitraClip® System Show Continued Benefit for People with Mitral Regurgitation, Most Common Heart Valve Disease
WASHINGTON, March 18, 2017 /PRNewswire/ — Abbott (ABT) today announced favorable one-year outcomes from the largest study of real-world experience for the MitraClip system in transcatheter mitral valve repair (TMVR) procedures in the United States. MitraClip treats people with degenerative mitral regurgitation (DMR, also known as leaky heart valve), a […]