– New results demonstrate MitraClip™ transcatheter edge-to-edge repair (TEER) is effective at treating leaky valves in people with secondary mitral regurgitation (MR) – Data from the first report on patients with a pacemaker lead across the tricuspid valve show TriClip™ and TriClip G4 TEER systems significantly reduce tricuspid regurgitation (TR) […]
Tag: Abbott
New Late-Breaking Data Highlight Abbott Structural Heart Transcatheter Valve Therapies
Data presented at EuroPCR 2022 reinforce the impact of Abbott’s minimally invasive heart devices on patient outcomes and quality of life Findings demonstrate TriClip™ transcatheter edge-to-edge (TEER) repair is effective at reducing tricuspid regurgitation across a broad range of anatomies One-year outcomes for Navitor™ transcatheter aortic valve implantation (TAVI) system […]
Study Finds 89% of Patients Treated for Persistent Atrial Fibrillation Using Abbott’s Ablation Device Remain Symptom-Free for at Least 15 Months
Treatment with the TactiCath™ Contact Force Ablation Catheter, Sensor Enabled™ resulted in patients with persistent atrial fibrillation experiencing an improved quality of life The catheter is part of Abbott’s suite of electrophysiology solutions designed to improve procedures to address cardiac arrythmias ABBOTT PARK, Ill., April 29, 2022 /PRNewswire/ — Abbott (NYSE: ABT) today […]
Abbott Strengthens Left Atrial Appendage Closure Leadership With U.S. Availability of Amplatzer™ Steerable Delivery Sheath for the Company’s Amulet™ Device
First and only steerable delivery system designed to help seal the left atrial appendage (LAA) in people with atrial fibrillation who are at an increased risk of stroke Now available in the U.S., Abbott’s steerable sheath offers precise and flexible placement when used with the company’s Amplatzer Amulet LAA Occluder […]
Abbott Reports First-Quarter 2022 Results
Sales growth of 13.8 percent; organic sales growth of 17.5 percent Global COVID-19 testing-related sales of $3.3 billion in the first quarter Excluding COVID-19 testing-related sales, first-quarter reported sales growth of 3.9 percent and organic sales growth of 7.7 percent GAAP diluted EPS growth of 37.0 percent; adjusted diluted EPS growth of […]
ABBOTT RECEIVES FDA APPROVAL FOR AVEIR™ VR LEADLESS PACEMAKER SYSTEM TO TREAT PATIENTS WITH SLOW HEART RHYTHMS
Abbott’s Aveir single chamber (VR) pacing system is the world’s only leadless pacemaker with a unique mapping capability to assess correct positioning prior to placement Aveir VR has an increased projected battery life that can be up to two times longer than other commercially available leadless pacemakers when using the […]
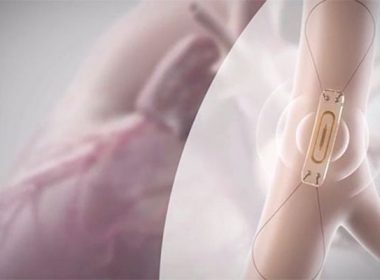
Abbott’s CardioMEMS™ HF System Receives FDA Approval to Support Patients Battling Earlier-Stage Heart Failure
– New expanded indication provides patients suffering from earlier stages of heart failure access to the CardioMEMS™ HF System, a small implantable sensor that can flag early warning signs of worsening heart failure – FDA approval was supported by data from the GUIDE-HF trial, which suggested that the CardioMEMS sensor […]
Abbott Announces World’s First Implant of Dual-Chamber Leadless Pacemaker in Pivotal Trial
– Abbott’s investigational Aveir™ DR dual-chamber pacemaker is designed to provide synchronous, beat-by-beat pacing of the right atrium and right ventricle of the heart – Proprietary implant-to-implant (i2i™) device technology is used for communication between two implanted leadless pacemakers to regulate the heart rate – Aveir DR is also specifically […]
Abbott Reports Strong Fourth-Quarter 2021 Results; Issues 2022 Forecast
– Fourth-quarter sales growth of 7.2 percent; organic sales growth of 7.7 percent – Global COVID-19 testing-related sales of $2.3 billion in the fourth quarter – Excluding COVID-19 testing-related sales, fourth-quarter sales growth of 9.6 percent and organic sales growth of 10.3 percent – Full-year 2021 sales growth of 24.5 […]
Abbott Receives U.S. FDA Clearance for New Cardiac Mapping System to Improve How Doctors Treat Abnormal Heart Rhythms
– EnSite™ X EP System with EnSite Omnipolar Technology (OT) provides a 360-degree view of the heart, regardless of catheter orientation, for cardiac mapping without compromise ABBOTT PARK, Ill., Jan. 12, 2022 /PRNewswire/ — Abbott (NYSE: ABT) today announced it has received clearance from the U.S. Food and Drug Administration for the EnSite™ X EP System […]