FY 2022 Annual Revenue of $1.032 billion, Up 22% Versus Prior Year DANVERS, Mass.–(BUSINESS WIRE)–ABIOMED, Inc. (NASDAQ: ABMD), a leader in breakthrough heart, lung and kidney support technologies, today announced financial results for the quarter and fiscal year ended March 31, 2022. Financial summary and operational highlights: Revenue for the […]
Tag: Abiomed
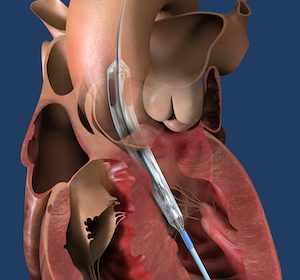
World’s First Patient Implanted with Impella BTR Minimally Invasive Heart Pump
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (Nasdaq: ABMD) announces the first patient in the world has been successfully implanted with Impella Bridge-to-Recovery (BTR) as part of the heart pump’s U.S. Food and Drug Administration (FDA) Early Feasibility Study (EFS). The first implant was done by Duc Thinh Pham, MD, and Jane Wilcox, MD, at […]
Abiomed Announces Record Revenue of $261 Million, up 13% Year Over Year
Increasing Low End of Guidance: $1,025 Million – $1,030 Million, up 21% – 22% DANVERS, Mass.–(BUSINESS WIRE)–ABIOMED, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart support technologies, today announced financial results for the quarter ended December 31, 2021. Q3 financial summary and operational highlights: Revenue for the quarter totaled […]
Early Feasibility Study Demonstrates Successful Use of Abiomed’s preCARDIA Technology
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces the successful results of the first-in-human early feasibility study of the preCARDIA system. The preCARDIA system is designed to improve decongestion in acutely decompensated heart failure (ADHF) patients by intermittently occluding the superior vena cava. The results of the study published this week in the […]
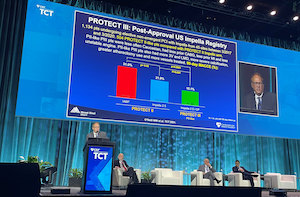
Two Large Studies Demonstrate Complete Revascularization with Impella Heart Pumps Improves Ejection Fraction and Long-Term Patient Outcomes
ORLANDO, Fla.–(BUSINESS WIRE)–The final results of the PROTECT III and Restore EF prospective studies demonstrate improved outcomes for high-risk PCI patients with the use of Impella heart pumps. The study results were reviewed this morning at Transcatheter Cardiovascular Therapeutics (TCT) 2021, the annual scientific symposium of the Cardiovascular Research Foundation, by Gregg […]
Abiomed Announces Q2 FY 2022 Revenue of $248 Million, Up 18% Year Over Year
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ:ABMD), a leading provider of breakthrough heart support technologies, today announced financial results for the quarter ended September 30, 2021. Q2 financial summary and operational highlights: Revenue for the quarter totaled $248.1 million, an increase of 18% compared to $209.8 million during the same period of […]
FDA Grants Breakthrough Device Designation to Impella ECP, the World’s Smallest Heart Pump
DANVERS, Mass.–(BUSINESS WIRE)–The United States Food and Drug Administration (FDA) has granted breakthrough device designation to Abiomed’s (NASDAQ: ABMD) Impella ECP expandable percutaneous heart pump. The designation means the FDA will prioritize Impella ECP’s regulatory review processes including design iterations, clinical study protocols and pre-market approval (PMA) application. Impella ECP is the smallest […]
Abiomed Announces Record Revenue of $253 Million, Up 53% Year Over Year
Full year global revenue guidance increased for fiscal year 2022 to 22% – 24% growth versus prior year DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart support technologies, today announced financial results for the quarter ended June 30, 2021. Q1 financial summary and operational highlights: Total […]
FDA Grants Highest Level of Approval to the Next Generation of Impella RP to Treat Right Heart Failure
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed’s (NASDAQ: ABMD) newest right heart pump, the Impella RP with SmartAssist, has received U.S. Food and Drug Administration (FDA) pre-market approval (PMA) as safe and effective to treat acute right heart failure for up to 14 days. Impella RP with SmartAssist is the first single-access temporary percutaneous ventricular […]
Abiomed Acquires preCARDIA, a Breakthrough Medical Device Company, to Improve Outcomes for Heart Failure Patients
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) has acquired preCARDIA, developer of a proprietary catheter and controller that will complement Abiomed’s product portfolio to expand options for patients with acute decompensated heart failure (ADHF). The preCARDIA system is uniquely designed to rapidly treat ADHF-related volume overload by effectively reducing cardiac filling pressures and […]