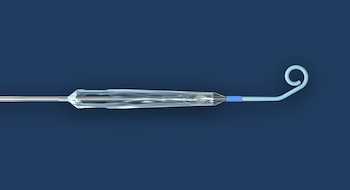
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces the achievement of two milestones in the development of small bore access for the Impella heart pump. The Impella ECP heart pump has completed the first stage in its U.S. Food and Drug Administration (FDA) early feasibility study (EFS) and the FDA has granted […]
Tag: Abiomed
First 1,000 Patients Treated with Impella 5.5 with SmartAssist, a Heart Pump Designed for Surgeons
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces 1,000 patients have been treated with the Impella 5.5 with SmartAssist heart pump in the first year after the U.S. Food and Drug Administration (FDA) granted Impella 5.5 with SmartAssist its highest level of approval for safety and efficacy. In October 2019, the first U.S. patients were […]
Abiomed Announces Q2 FY 2021 Revenue of $210 Million, Up 27% Over Q1 FY 2021 and Up 2% Over Q2 FY 2020 With 29.2% Operating Margin
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart support technologies today reported second quarter fiscal 2021 revenue of $209.8 million, a sequential increase of 27% compared to Q1 fiscal year 2021 and a year over year increase of 2% compared to Q2 fiscal year 2020 despite […]
First Patients Treated with the World’s Smallest Heart Pump, the 9Fr Impella ECP
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces the first two patients have been treated with the Impella ECP expandable percutaneous heart pump. Impella ECP is the smallest heart pump in the world. It measures 9 French (Fr) (3 millimeters) in diameter upon insertion and removal from the body. While in the heart, […]
FDA Grants 510(k) Clearance for Abiomed’s Innovative Cardiopulmonary Support Technology
DANVERS, Mass.–(BUSINESS WIRE)–The United States Food and Drug Administration (FDA) has granted Abiomed (NASDAQ: ABMD) a 510(k) clearance for an all-in-one, compact cardiopulmonary bypass system called the Abiomed Breethe OXY-1 System™. The ECMO system provides cardiopulmonary bypass support for patients whose lungs can no longer provide sufficient end organ oxygenation. The 510(k) clearance […]
Data Presented at TCT Connect Finds Pre-PCI Use of Impella for AMI Cardiogenic Shock is Associated with Higher Survival, Particularly in Women
DANVERS, Mass.–(BUSINESS WIRE)–Two studies of AMI cardiogenic shock (AMICS) patients found higher survival when Impella was placed pre-PCI, compared to when Impella was placed after PCI. The findings were presented at TCT Connect, the 32nd annual scientific symposium of the Cardiovascular Research Foundation. In the first study, presented by Hemindermeet Singh, […]
Largest Study of Hemodynamically Supported High-Risk PCI Patients Finds More Complete Revascularization with Impella Leads to Improved Outcomes
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces new PROTECT III study data that demonstrates reduced rates of MACCE (composite of death, stroke, myocardial infarction and repeat procedures) when Impella is used to achieve a more complete revascularization in a single setting for high-risk percutaneous coronary intervention (PCI) patients. PROTECT III is an ongoing, prospective, […]
Restore EF Study Demonstrates Impella-Supported High-Risk PCI Improves Left Ventricular Ejection Fraction
DANVERS, Mass.–(BUSINESS WIRE)–The Restore EF Study demonstrates the use of contemporary best practices, including attempting a more complete revascularization with Impella-supported high-risk PCI, is associated with significant improvement of left ventricular ejection fraction (LVEF), heart failure symptoms, and anginal symptoms at follow up. The interim analysis was presented today by Mitul […]
TCT Connect to Highlight How Impella Enables Improved Outcomes for High-Risk PCI, Cardiogenic Shock and Right Heart Failure Patients
DANVERS, Mass.–(BUSINESS WIRE)–The benefits of a more complete revascularization with Impella heart pumps in high-risk percutaneous coronary intervention (PCI) patients and the value of Impella protocol-based treatment for survival and native heart recovery in cardiogenic shock patients will be highlighted at TCT Connect, the 32nd annual scientific symposium of the Cardiovascular Research Foundation. […]
Abiomed Announces Q1 FY 2021 Revenue of $165 Million and 21% Operating Margin
June Revenue Up 4% Versus Prior Year DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart support technologies today reported first quarter fiscal 2021 revenue of $164.9 million compared to revenue of $207.7 million for the same period of fiscal 2020 despite a negative impact from the […]