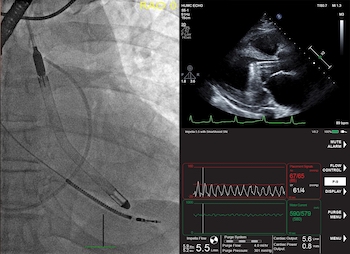
DANVERS, Mass.–(BUSINESS WIRE)–The United States Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for left-sided Impella heart pumps to provide left ventricular unloading and support to COVID-19 patients who are undergoing ECMO treatment and develop pulmonary edema or myocarditis. Impella is manufactured by Abiomed (NASDAQ: ABMD). COVID-19 causes widespread inflammation which can […]
Tag: Abiomed
Large Multi-Center Study in Japan Finds High Survival Rates with Use of Impella Heart Pump
Study conducted at 109 hospitals with oversight by 10 Japanese professional societies, including Japanese Circulation Society TOKYO–(BUSINESS WIRE)–A three-year, investigator-led, prospective study of Japanese patients who received an Impella heart pump finds use of Impella is associated with a 77% survival rate at 30 days in AMI cardiogenic shock patients. The historic […]
Abiomed First Quarter Fiscal 2021 Earnings and Conference Call Notification
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ: ABMD) announced that on Thursday, August 6, 2020, the Company will release financial results for the first quarter of fiscal 2021. The Company will host a conference call to discuss the results on Thursday, August 6, 2020 at 8:00 a.m. ET. Michael R. Minogue, Chairman, […]
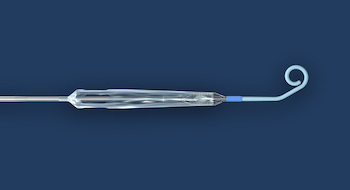
Study Finds 84% Survival Rate in Patients in Cardiogenic Shock and Other Challenging Cardiac Conditions with the New Impella 5.5 with SmartAssist
DANVERS, Mass.–(BUSINESS WIRE)–The first published United States experience of patients who received Abiomed’s newest heart pump, Impella 5.5 with SmartAssist, finds 84% of the patients survived to explant with 76% native heart recovery. The study was published in the July edition of the American Society for Artificial Internal Organs (ASAIO) Journal. The study examined […]
Abiomed Launches Virtual Physician Education Program, CAMP PCI, to Improve High-Risk PCI Patient Outcomes
DANVERS, Mass.–(BUSINESS WIRE)–Today, Abiomed (NASDAQ: ABMD) launches a cutting-edge digital education platform called CAMP PCI, which stands for Coronary Artery & Myocardial Protected Percutaneous Coronary Intervention, to improve patient outcomes and quality of life with supported high-risk PCI by utilizing best practices, techniques and technologies to enable safer, more effective and complete revascularization. […]
Abiomed Appoints New Chief Medical Officer Charles Simonton, M.D.
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (Nasdaq: ABMD), maker of the Impella heart pump, announced today it has appointed Charles (Chuck) Simonton, M.D., as vice president and chief medical officer. Dr. Simonton is renowned for his leadership in product development and large-scale clinical trials. He was most recently at Abbott Vascular where he served as chief medical officer […]
FDA Approves Abiomed’s First-in-Human Trial of Impella ECP, World’s Smallest Heart Pump
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces the United States Food and Drug Administration (FDA) has approved the company’s investigational device exemption application to start an early feasibility study with a first-in-human trial of the 9 French (Fr) Impella ECP™ heart pump. Impella ECP, which stands for expandable cardiac power, will be […]
FDA Issues Emergency Use Authorization for Impella RP as Therapy for COVID-19 Patients with Right Heart Failure
DANVERS, Mass.–(BUSINESS WIRE)–The United States Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for Impella RP to include patients suffering from COVID-19 related right heart failure or decompensation, including pulmonary embolism (PE). Abiomed (NASDAQ: ABMD) manufactures Impella RP. Impella RP is a temporary heart pump that provides circulatory support for […]
PROTECT III Study Shows Placing Impella Prior to High-Risk PCI is Associated with Lower Mortality Compared to Bailout PCI
Women Disproportionately Impacted with Bailout DANVERS, Mass.–(BUSINESS WIRE)–Data from more than 1,000 patients presented during the virtual 2020 Society for Cardiovascular Angiography & Interventions (SCAI) Scientific Sessions demonstrates Impella reduced in-hospital mortality when placed before a non-emergent percutaneous coronary intervention (PCI) is performed. As detailed in the online presentation, the research found, in […]
Abiomed Expands Product Portfolio with Acquisition of Cardiopulmonary Support Technology (ECMO) to Improve Outcomes for Patients
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD), maker of the Impella heart pump, has acquired Breethe, developer of a novel extracorporeal membrane oxygenation (ECMO) system that will complement and expand Abiomed’s product portfolio to more comprehensively serve the needs of patients whose lungs can no longer provide sufficient oxygenation, including patients suffering from cardiogenic shock […]