FY 2020 Annual Revenue of $841 Million, Up 9%, and 29.6% Operating Margin DANVERS, Mass.–(BUSINESS WIRE)–Apr. 30, 2020– Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart support technologies today reported fourth quarter fiscal 2020 revenue of $206.7 million compared to revenue of $207.1 million for the same period of fiscal 2019. For fiscal year 2020, […]
Tag: Abiomed
Abiomed’s Response to the COVID-19 Pandemic
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD), maker of the Impella heart pump, is taking a number of steps to aid the global medical community and contribute to improved patient care during the COVID-19 outbreak. Impella heart pumps are FDA approved to provide circulatory support to allow the heart to rest and recover for patients […]
Abiomed Announces Q3 FY 2020 Revenue of $222 Million and 31.7% Operating Margin
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart recovery and support technologies, today reported third quarter fiscal 2020 revenue of $221.6 million, an increase of 10% compared to revenue of $200.6 million for the same period of fiscal 2019. Operating income was $70.3 million, up 13%, compared […]
Abiomed Announces Preliminary Q3 FY 2020 Revenue of $222 Million, up 10% Over Prior Year
SAN FRANCISCO–(BUSINESS WIRE)–Jan. 13, 2020– Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart support technologies today reported preliminary, unaudited, third quarter fiscal 2020 revenue of approximately $221.6 million, an increase of 10% compared to revenue of $200.6 million for the same period of fiscal 2019. Abiomed had a strong start to the quarter across […]
Large Randomized Controlled Trial Aims to Curb Rising Epidemic of Heart Failure
BOSTON–(BUSINESS WIRE)–A pivotal, multi-center clinical trial to explore a promising new therapy to reduce heart failure rates by changing the way heart attack patients are treated is now underway. The first patient has enrolled in the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial. The first enrollment took […]
First Patient Enrolls in STEMI DTU Randomized Controlled FDA Trial; Study Aims to Further Demonstrate Impella’s Safety and Effectiveness
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces initiation of the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial (RCT), which will explore whether unloading the heart’s left ventricle for 30 minutes with an Impella heart pump prior to opening blocked arteries will reduce infarct size after a heart attack and lead to […]
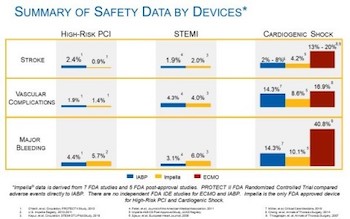
Clinical Review Demonstrates Cost-Effectiveness of Impella in High-Risk PCI and Cardiogenic Shock
DANVERS, Mass.–(BUSINESS WIRE)–To mark the five year anniversary of the study by Stretch, et al., on cost and outcomes trends for short-term mechanical circulatory support, Abiomed announces a comprehensive publication review of cost and comparative effectiveness of Impella in high-risk PCI and cardiogenic shock. The data, from a robust body of US and European […]
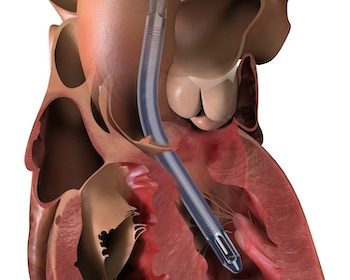
First U.S. Patients Treated with Impella 5.5 with SmartAssist, a Minimally Invasive, Forward Flow Heart Pump
DANVERS, Mass.–(BUSINESS WIRE)–Three cardiac surgeons at Cleveland Clinic, Hackensack Meridian Health and Cedars-Sinai Medical Center are the first in the United States to implant Abiomed’s (NASDAQ: ABMD) newest heart pump, the Impella 5.5 with SmartAssist. Ed Soltesz, MD, Mark Anderson, MD, and Danny Ramzy, MD, have each successfully implanted multiple pumps during cardiac procedures at their hospitals. Mark […]
Abiomed Announces Q2 FY 2020 Revenue of $205 Million and 29.4% Operating Margin
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart recovery and support technologies, today reported second quarter fiscal 2020 revenue of $205.0 million, an increase of 13% compared to revenue of $181.8 million for the same period of fiscal 2019. Operating income was $60.2 million, up 20%, compared […]
CORRECTING and REPLACING CAPTION FDA Post Approval Study Demonstrates Timely Identification of Right Heart Failure and Early Use of Impella RP Leads to Higher Survival
CORRECTION…by Abiomed October 26, 2019 09:15 AM Eastern Daylight Time DETROIT–(BUSINESS WIRE)–Please replace the caption for release dated October 25, 2019 with the accompanying corrected caption. The release reads: FDA POST APPROVAL STUDY DEMONSTRATES TIMELY IDENTIFICATION OF RIGHT HEART FAILURE AND EARLY USE OF IMPELLA RP LEADS TO HIGHER SURVIVAL […]