TOKYO–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD), a leading provider of breakthrough heart support technologies, announces the 1,000th patient has been treated with the Impella heart pump in Japan. The Impella 2.5 and Impella 5.0 heart pumps are approved for the treatment of drug-resistant acute heart failure and are the first and only percutaneous temporary […]
Tag: Abiomed
Abiomed Receives FDA PMA Approval for Impella 5.5 with SmartAssist, a Minimally Invasive, Forward Flow Heart Pump
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed’s (NASDAQ: ABMD) newest heart pump, the Impella 5.5 with SmartAssist, has received U.S. Food and Drug Administration (FDA) pre-market approval (PMA) for safety and efficacy in the therapy of cardiogenic shock for up to 14 days. Impella 5.5 with SmartAssist is: Minimally invasive, eliminating the need for a […]
Highest Court in Germany Affirms Strength of Abiomed’s Patents
DANVERS, Mass.–(BUSINESS WIRE)–The highest court in Germany, the Federal Court of Justice, recently ruled in favor of Abiomed (NASDAQ: ABMD) in a patent challenge filed by Thoratec in 2015 and validated the strengths of Abiomed’s Impella-related patents. These patents are also the subject of a separate patent infringement action Abiomed […]
Impella® SmartAssist Platform Launches at ESC, Designed to Further Improve Patient Outcomes
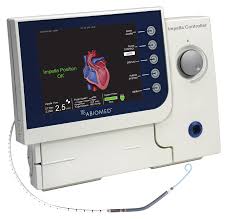
The new Impella CP heart pump features a fiber optic sensor, optimally positioned to measure the placement signal in the aorta, identify pump placement and enable repositioning without the use of imaging. (Graphic: Abiomed) PARIS–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces today that the Impella CP® with SmartAssist technology, which is designed to improve patient […]
Pomerantz Law Firm Announces the Filing of a Class Action against ABIOMED, Inc. and Certain Officers – ABMD
NEW YORK, Aug. 06, 2019 (GLOBE NEWSWIRE) — Pomerantz LLP announces that a class action lawsuit has been filed against ABIOMED, Inc. (“ABIOMED” or the “Company”) (NASDAQ: ABMD) and certain of its officers. The class action, filed in United States District Court, for the Southern District of New York, and […]
Abiomed Announces Q1 FY 2020 Revenue of $208 Million and 29.2% Operating Margin
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart recovery and support technologies, today reported first quarter fiscal 2020 revenue of $207.7 million, an increase of 15.4% compared to revenue of $180.0 million for the same period of fiscal 2019. Operating income was $60.7 million, up 30%, compared […]
National Cardiogenic Shock Initiative (NCSI) with Impella Best Practices Demonstrates 72% Survival with 98% Native Heart Recovery
LAS VEGAS–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) announces that new data from the National Cardiogenic Shock Initiative Study (NCSI) on 171 consecutive AMI cardiogenic shock (AMICS) patients from 35 sites demonstrates 72% survival with 98% native heart recovery at discharge. The patients were treated with the NCSI protocol, which includes placing the Impella heart pump before […]
Impella SmartAssist Platform Launches at SCAI, Designed to Further Improve Patient Outcomes
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) announces today that the Impella CP with SmartAssist, which is designed to improve patient outcomes with advanced algorithms and simplified patient management, will be commercially available beginning at the 2019 Society for Cardiovascular Angiography & Interventions (SCAI) Scientific Sessions through a controlled launch process at select sites. The […]
FDA Approves Impella 5.0 and Impella LD Extended Duration of Use to 14 Days for Cardiogenic Shock Derived from AMI or Cardiomyopathy
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) announces the U.S. FDA has approved the expansion of the Impella 5.0 and Impella LD PMA labeling for the treatment of cardiogenic shock. The expansion extends the duration of support for each pump from 6 days to 14 days. The Impella 5.0 and the Impella LD are forward flow heart pumps […]
Abiomed Announced Q4 FY 2019 Revenue of $207 Million and 31.6% Operating Margin
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart recovery and support technologies, today reported fourth quarter fiscal 2019 revenue of $207.1 million, an increase of 19% compared to revenue of $174.4 million for the same period of fiscal 2018. For fiscal year 2019, total revenue was $769.4 […]