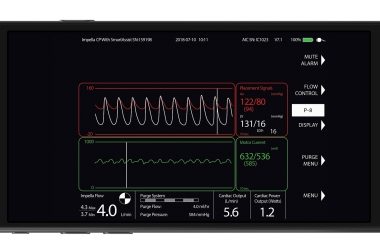
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces that, on April 26, the FDA approved initiation of the ST-Elevation Myocardial Infarction Door-to-Unloading (STEMI DTU) Pivotal Randomized Controlled Trial. The prospective, multi-center, two-arm trial plans to enroll 668 patients undergoing treatment for a STEMI heart attack. Half the patients will be randomized to receive […]
Tag: Abiomed
OPSENS SIGNS LANDMARK SUPPLIER AGREEMENT – EXPANDS OPTICAL SENSING TECHNOLOGY IMPACT WITHIN CARDIOLOGY SEGMENT
Quebec City, Quebec, April 30, 2019 – Opsens inc. (“Opsens” or the “Company”) (TSX:OPS) (OTCQX:OPSSF) is pleased to announce it has entered into a supply agreement as part of its long-term collaboration with Abiomed, Inc. (“Abiomed”) for the Impella CP® heart pump. Abiomed has awarded Opsens a five-year agreement to supply […]
Cardiogenic Shock Survival Rates Improve Significantly in Three Years Since Impella FDA PMA Approval
DANVERS, Mass.–(BUSINESS WIRE)–Three years ago this week, Abiomed’s (NASDAQ: ABMD) Impella heart pumpreceived its FDA PMA approval for AMI cardiogenic shock. At the time of Impella’s FDA PMA approval, the cardiogenic shock survival rate to explant in the Impella Quality Assurance (IQ) Database was 51% in the United States1. Today, Impella heart pumps, combined […]
Impella RP Post-Approval Study Data Presented at ACC 2019
March 18, 2019 07:00 AM Eastern Daylight Time NEW ORLEANS, La.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD), a leading provider of breakthrough heart support technologies and the maker of the Impella RP heart pump, announces that survival data from the 18 month post-approval study of 42 Impella RP patients was presented at the American College of Cardiology’s […]
$100 Million Invested in Clinical Research for Impella
DANVERS, Mass.–(BUSINESS WIRE)–In a milestone for scientific research, Abiomed (NASDAQ: ABMD) has now invested more than $100 million over the past five years in clinical research on the Impella heart pump platform. Abiomed’s commitment to clinical research is detailed on a new webpage that launched today. Abiomed-sponsored research is augmented by two decades of independent physician-led […]
Impella Connect Achieves Regulatory Milestone
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) has achieved CE Mark for Impella Connect, the first-of-its kind cloud-based technology that enables secure, real-time, remote viewing of the Impella console for physicians and hospital staff from anywhere with Internet connectivity. European CE Mark adds to Impella Connect’s previous U.S. FDA PMA approval. Impella Connect uses […]
Abiomed Announces Q3 FY 2019 Record Revenue of $201 Million, up 30% over Prior Year
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart recovery and support technologies, today reported third quarter fiscal 2019 revenue of $200.6 million, an increase of 30% compared to revenue of $154.0 million for the same period of fiscal 2018. Third quarter fiscal 2019 GAAP net income was […]
Abiomed to Present at the 37th Annual J.P. Morgan Healthcare Conference
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed, Inc. (NASDAQ: ABMD), a leading provider of breakthrough heart support technologies, today announced that Michael R. Minogue, Chairman, President and Chief Executive Officer, will present at the 37th Annual J.P. Morgan Healthcare Conference on Monday, January 7, 2019 at 5:00 p.m. PST / 8:00 p.m. EST. The […]
Shockwave Announces collaboration With Abiomed on Physician Training
SANTA CLARA, Calif., Dec. 11, 2018 (GLOBE NEWSWIRE) — Shockwave Medical, a pioneer in the development and commercialization of intravascular lithotripsy to treat complex calcified cardiovascular disease, today announced an investment and collaboration agreement with Abiomed, Inc. As outlined by the agreement, Abiomed will invest $15 million in Shockwave and […]
FDA Safety Study of Unloading the Left Ventricle for 30 Minutes Prior to Reperfusion in Heart Attack Patients is Safe and Feasible
CHICAGO–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces the results of the FDA STEMI Door-to-Unloading safety and feasibility randomized controlled trial, which show unloading the left ventricle with Impella CP® for 30 minutes prior to reperfusion in patients presenting with anterior ST-segment elevation myocardial infarction (STEMI) without cardiogenic shock is safe and feasible, when compared to Impella […]