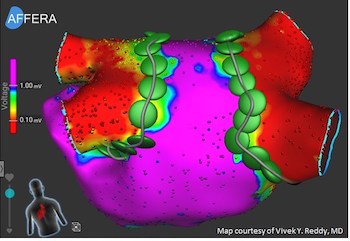
First of its kind, all-in-one Sphere-9™ Catheter with pulsed field ablation, radiofrequency, and high density mapping integrated with intuitive mapping and navigation platform DUBLIN, March 15, 2023 /PRNewswire/ — Medtronic (NYSE:MDT) announced today that it has received CE (Conformité Européenne) Mark for the Affera™ Mapping and Ablation System, which includes the Sphere-9™ […]
Tag: Affera
Medtronic completes enrollment in pivotal trial evaluating first-of-its-kind pulsed field ablation catheter for patients with atrial fibrillation
SPHERE Per-AF will determine the safety and effectiveness of the Sphere-9 cardiac ablation and mapping catheter with the Affera mapping and navigation system DUBLIN, Dec. 5, 2022 /PRNewswire/ — Medtronic (NYSE:MDT) today announced the completion of enrollment and final treatment in the SPHERE Per-AF Trial, a U.S. Food and Drug Administration (FDA) […]
Medtronic completes acquisition of Affera
Acquisition adds mapping and navigation platform to company’s cardiac ablation portfolio and brings an integrated system of diagnostic and therapeutic tools to address a broad spectrum of arrhythmias DUBLIN, Aug. 30, 2022 /PRNewswire/ — Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced that it has completed the acquisition of […]
Medtronic to Acquire Affera
Acquisition to expand company’s cardiac ablation portfolio, including first-time entry into mapping and navigation, within one of the fastest growing medtech markets DUBLIN, Jan. 10, 2022 /PRNewswire(opens new window)/ — Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced that it has entered into a definitive agreement to acquire Affera, Inc., […]
Affera Announces First Patient Treated in SPHERE Per-AF IDE Trial
First enrollment comes as investors provide $75M toward an oversubscribed Series C financing NEWTON, Mass., Dec. 20, 2021 /PRNewswire/ — Affera Inc., a private medical device company focused on innovative cardiac arrhythmia treatment solutions, announced today that the first patient was treated in the recently approved SPHERE PerAF Trial, a U.S. Food and […]
Affera Announces World’s First Successful Focal Pulsed Field Ablation in Patients
WATERTOWN, Mass., Dec. 19, 2019 /PRNewswire/ — Affera, Inc., a private medical device company focused on innovative cardiac arrhythmia treatment solutions, announced today that its focal Pulsed Field (“PF”) ablation technology, also known as Irreversible Electroporation (“IRE”), has been successfully used to treat 40 patients suffering from Atrial Fibrillation (“AFIB”). This multi-center […]
Affera’s Novel Technology For Cardiac Arrhythmia Treatment To Be Featured In 8 Scientific Sessions At The 2018 Heart Rhythm Society Meeting
WATERTOWN, Mass., May 7, 2018 /PRNewswire/ — Affera, Inc, a private medical device company focused on innovative cardiac arrhythmia treatment solutions, announced today its integrated platform will be highlighted in eight scientific sessions at the Heart Rhythm Society 2018 (HRS) meeting in Boston, Massachusetts. Affera’s revolutionary lesion formation technology offers a solution designed to optimize procedural efficiency […]