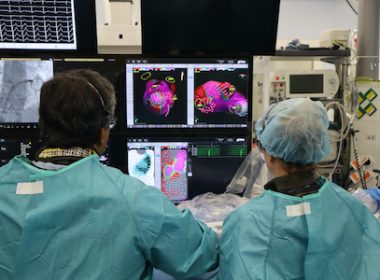
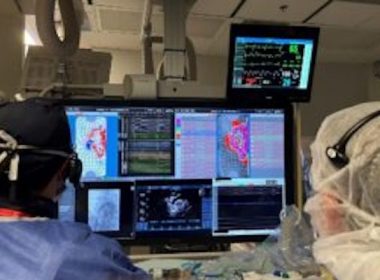
VANCOUVER, British Columbia–(BUSINESS WIRE)–Kardium Inc. announces the successful first-in-human study of its next generation Globe® Pulsed Field System to treat atrial fibrillation (AF) using pulsed field ablation (PFA) therapy. Working with Dr. Vivek Reddy1 from Mount Sinai Hospital (New York, USA), and Prof. Petr Neužil and Dr. Jan Petrů from Na Homolce […]
Tag: AFib
AbbVie Announces Late-Breaking Results from Phase 2 Exploratory NOVA Trial of Novel Investigational Neurotoxin AGN-151607 for the Prevention of Postoperative Atrial Fibrillation in Cardiac Surgery Patients
Primary endpoint was not met for the modified intent-to-treat (mITT) population; however, clinical benefit observed in coronary artery bypass graft (CABG) patients Data showed relative risk reduction in specific study populations and overall lower rates of rehospitalization within 30 days compared to placebo Adverse Events (AEs) were numerically similar across […]
Volta Medical’s AI based solution for patients in atrial fibrillation achieves robust validation in first peer-reviewed publication
Volta VX1 decision support software reproduces expert-physician electrogram (EGM) analysis that can assist physicians in the real-time identification of specific abnormal electrograms in a persistent atrial fibrillation (AF) population After a one-year follow-up, patients demonstrated a high freedom from atrial fibrillation and from any atrial arrhythmia, 89% and 73% after […]
CardioFocus Announces Pulsed Field Ablation Milestones
MARLBOROUGH, Mass., July 19, 2022 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatments for atrial fibrillation (Afib), today announced several milestones toward the development of a next generation pulse field ablation (PFA) technology for treatment of AFib, including the formation of a clinical advisory board, filing of […]
Study Demonstrates Cardiologs’ AI Can Predict Occurrence of Atrial Fibrillation in the Near Future
Newly published research shows that Cardiologs’ deep learning model can predict the short-term risk of atrial fibrillation (AFib) based on 24-hour Holter recordings that show normal sinus rhythm. PARIS and BOSTON, June 21, 2022 /PRNewswire/ — Cardiologs, a global leader in artificial intelligence (AI) cardiology diagnostics, announced today that its latest study, “Short-term prediction of atrial […]
AtriCure Announces the First Patient Treated in the HEAL-IST Clinical Trial
Trial will evaluate the safety and effectiveness of AtriCure’s Isolator® Synergy™ Clamp in conjunction with endocardial catheter ablation for the treatment of Inappropriate Sinus Tachycardia MASON, Ohio–(BUSINESS WIRE)–AtriCure, Inc. (Nasdaq: ATRC), a leading innovator in surgical treatments and therapies for atrial fibrillation (Afib), left atrial appendage (LAA) management and post-operative pain […]
Kardium’s Globe® System Begins Clinical Trial at St. Paul’s Hospital
A revolutionary device that treats Atrial Fibrillation (AF) – the world’s most common heart rhythm disorder – helps support the healthy aging of BC’s population. April 12, 2022, 9:00 AM Pacific Daylight Time | VANCOUVER, British Columbia – Kardium Inc. announces a successful first-in-BC investigational* clinical procedure using the Globe® Mapping and Ablation […]
Zio® by iRhythm Shown to Prevent Hospital Admissions, Aid in Early Detection of AF
New clinical research presented at ACC.22 shows that the Zio service is a viable solution for the early detection of silent atrial fibrillation Additional data shows that Zio AT can positively impact hospital resources – saving one healthcare system 136 inpatient hospitalization days SAN FRANCISCO, April 03, 2022 (GLOBE NEWSWIRE) — iRhythm […]
AtriCure Publishes Inaugural ESG Report
MASON, Ohio–(BUSINESS WIRE)–AtriCure, Inc. (Nasdaq: ATRC), a leading innovator in surgical treatments and therapies for atrial fibrillation (Afib), left atrial appendage (LAA) management and post-operative pain management, today published its inaugural Environmental, Social and Governance (ESG) report highlighting the company’s corporate responsibility and sustainability initiatives. AtriCure’s 2021 ESG Report showcases several […]
Carta Healthcare Announces Distinction as an NCDR Certified Software Vendor for the American College of Cardiology’s (ACC) AFib Ablation Registry
Atlas, Carta Healthcare’s AI-assisted data abstraction solution, provides seamless, end-to-end submissions to the ACC’s AFib Ablation clinical data registry SAN FRANCISCO–(BUSINESS WIRE)–Carta Healthcare, provider of solutions for common healthcare data challenges through a combination of people, processes, and artificial intelligence (AI)–powered technology, today announced that it is now a National […]