S4 Medical oversubscribes round in preparation for clinical studies COLUMBUS, Ohio, Feb. 13, 2020 /PRNewswire/ — S4 Medical, an innovative medical device company focused on protecting the esophagus from thermal damage during catheter ablation procedures, announced an oversubscribed round of funding following its successful human study. While catheter ablation is an effective […]
Tag: AFib
CardioFocus Announces U.S. FDA PMA Supplement Submission For The Breakthrough HeartLight X3 Endoscopic Ablation System
MARLBOROUGH, Mass., Feb. 4, 2020 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), today announced that the company has submitted a pre-market approval (PMA) supplement to the U.S. Food and Drug Administration (FDA) for the HeartLight X3 Endoscopic Ablation System for the treatment of AFib. This […]
Late-Breaking Clinical Study Presented at AF Symposium 2020 Evaluates Attune Medical’s ensoETM For Use During Cardiac Ablation Procedures
CHICAGO–(BUSINESS WIRE)–Chicago-based medical device firm Attune Medical announced the presentation of clinical results from the largest device study to date designed to evaluate the performance of its ensoETM patient temperature management device as an aid in the reduction of esophageal injury during cardiac ablation, a common treatment for atrial fibrillation. […]
FDA Clears Eko’s AFib and Heart Murmur Detection Algorithms, Making It the First AI-Powered Stethoscope to Screen for Serious Heart Conditions
Eko’s algorithms alert clinicians to the presence of heart murmurs and atrial fibrillation (AFib) during the physical exam, converting the classic stethoscope into a powerful early detection tool SAN FRANCISCO–(BUSINESS WIRE)–Eko, a digital health company applying artificial intelligence (AI) in the fight against heart disease, announced today that the U.S. […]
Correvio Receives Complete Response Letter From U.S. FDA for Brinavess and Provides Update on Recent Events
NASDAQ: CORV TSX: CORV VANCOUVER, Dec. 24, 2019 /PRNewswire/ – Correvio Pharma Corp. (NASDAQ: CORV) (TSX: CORV), a specialty pharmaceutical company focused on commercializing hospital drugs, today announced it has received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) regarding the New Drug Application (NDA) for Brinavess™ (vernakalant IV), an anti-arrhythmic […]
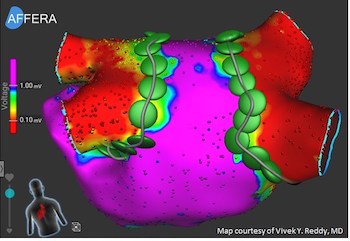
Affera Announces World’s First Successful Focal Pulsed Field Ablation in Patients
WATERTOWN, Mass., Dec. 19, 2019 /PRNewswire/ — Affera, Inc., a private medical device company focused on innovative cardiac arrhythmia treatment solutions, announced today that its focal Pulsed Field (“PF”) ablation technology, also known as Irreversible Electroporation (“IRE”), has been successfully used to treat 40 patients suffering from Atrial Fibrillation (“AFIB”). This multi-center […]
Adagio Medical Announces First Patient Enrollment in its iCLAS™ U.S. IDE Clinical Trial Evaluating Ultra-Low Temperature Cryoablation for Persistent Atrial Fibrillation
LAGUNA HILLS, Calif., Dec. 16, 2019 /PRNewswire/ — Adagio Medical, Inc. (Adagio) today announced it has enrolled the first patient in its iCLAS™ Investigational Device Exemption (IDE) trial. The FDA approved study aims to evaluate the safety and efficacy of Adagio’s ultra-low temperature intelligent Continuous Lesion Ablation System (iCLAS) in the treatment of persistent atrial fibrillation (PsAF). Data will […]
CardiaCare Awarded First Prize at the Innovation in Cardiovascular Interventions (ICI) Technology Parade in Tel Aviv, Israel
TEL AVIV, Israel, Dec. 16, 2019 /PRNewswire/ — CardiaCare, a startup developing wearable therapeutic devices for treating and monitoring AFib, announced today that it has been named the First Prize winner of the 2019 Innovation in Cardiovascular Interventions (ICI) Technology Parade in Tel Aviv. CardiaCare presented its innovative product, a wearable neuromodulation (nerve […]
Correvio Stock Trading Halted Today; FDA Advisory Committee Meeting To Discuss Brinavess™ For Recent Onset Atrial Fibrillation
NASDAQ: CORV TSX: CORV VANCOUVER, Dec. 10, 2019 /PRNewswire/ – Correvio Pharma Corp. (NASDAQ: CORV) (TSX: CORV), a specialty pharmaceutical company focused on commercializing hospital drugs, today announced that NASDAQ and the Toronto Stock Exchange (TSX) have halted trading of the Company’s common stock. The U.S. Food and Drug Administration (FDA)’s Cardiovascular and Renal […]
Biosense Webster Launches a Two-pronged Initiative to Tackle the New Millennium Epidemic of Atrial Fibrillation
A new Atrial Fibrillation Management Report highlights that catheter ablation is almost 10 times more effective at delaying Atrial Fibrillation progression than drug therapy, yet just 4% of eligible patients are receiving ablation therapy Get Smart About AFIB (GSAAF) is a new public health campaign launched to tackle the growing […]