MOUNTAIN VIEW, Calif., Feb. 10, 2022 /PRNewswire/ — AliveCor, the leading innovator in FDA-cleared personal electrocardiogram (ECG) technology, today announced the appointment of Patricia Baran as Senior Vice President, Healthcare Americas. In this role, Baran will report to Vincent Balsamo, Executive Vice President of Worldwide Sales. Baran will lead the Employer, Payor, and Health System business segments […]
Tag: AliveCor
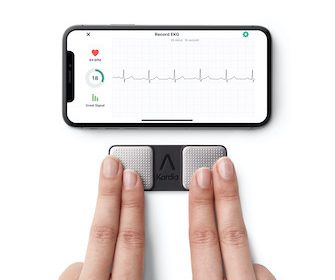
FDA Clears World’s First Credit-Card-Sized Personal ECG
AliveCor launches KardiaMobile Card, the only personal ECG slim enough to fit in a wallet for instant feedback on heart health anytime, anywhere MOUNTAIN VIEW, Calif., Feb. 1, 2022 /PRNewswire/ — AliveCor, the leading innovator in FDA-cleared personal electrocardiogram (ECG) technology, today announced the launch of KardiaMobile Card, the slimmest, most convenient personal […]
AliveCor Announces Strategic Additions to Executive Team
MOUNTAIN VIEW, Calif., Jan. 11, 2022 /PRNewswire/ — AliveCor, the global leader in FDA-cleared personal electrocardiogram (ECG) technology, today announced the addition of four new leaders to the company’s executive team: Vincent Balsamo as Executive Vice President of Worldwide Sales, Dr. Archana Dubey as Chief Clinical Officer, Dr. Ben Green as Senior Vice President of Services, and Jessica Weinstein as Senior […]
NICE recommends first smartphone-based ECG for the detection of atrial fibrillation (AF) for people with suspected paroxysmal AF in an ambulatory monitoring setting
AliveCor’s KardiaMobile® is the only personal electrocardiogram (ECG) to be recommended by the National Institute for Health and Care Excellence (NICE) for use within the NHS[1] NICE’s positive recommendation[1] offers patients and physicians across England and Wales access to a medical-grade ECG to support at home monitoring of paroxysmal atrial fibrillation (AF) without the requirements […]
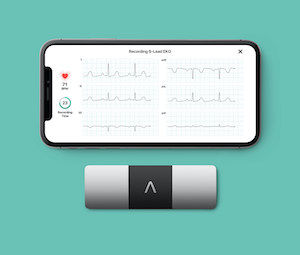
AliveCor Lists its Industry-leading KardiaMobile 6L in Epic’s App Orchard Marketplace AliveCor logo (PRNewsfoto/AliveCor, Inc.)
MOUNTAIN VIEW, Calif., Dec. 20, 2021 /PRNewswire/ — AliveCor, the global leader in FDA-cleared personal electrocardiogram (ECG) technology, today announced that KardiaMobile 6L, the most clinically-validated six-lead personal ECG device, is now available via KardiaPro on Epic’s App Orchard Marketplace for hospitals, health systems and providers. The integration will enable even more healthcare […]
LiveCare Partners with AliveCor to Launch Enhanced Remote Cardiac Monitoring Solution for Geriatric Patients at Home and in Skilled Nursing Facilities
NEW YORK, Dec. 7, 2021 /PRNewswire/ — LiveCare, a leader in geriatric monitoring technology, today announced the successful integration of AliveCor’s KardiaMobile® 6L, the world’s most clinically-validated personal electrocardiogram (EKG) device, within the LiveCare Link+ smart RPM gateway. Now, patients and their caregivers will receive real-time EKG monitoring at home or in skilled nursing […]
KardiaMobile 6L is Superior to One Lead Personal Devices
MOUNTAIN VIEW, Calif., Sept. 1, 2021 /PRNewswire/ — AliveCor, a leading innovator in FDA-cleared personal electrocardiogram (ECG) technology and services, today announced the KardiaMobile® 6L device was found to be superior to single lead devices according to results from the largest study to assess the reliability of the detection of atrial fibrillation (AF) through […]
New Study Demonstrates KardiaMobile 6L Reduces Urgent Healthcare Utilization
MOUNTAIN VIEW, Calif., July 27, 2021 /PRNewswire/ — A new study to be presented this week at Heart Rhythm 2021 and recently published in the American Journal of Cardiology1 shows use of the KardiaMobile 6L device results in a reduction of in-person healthcare utilization. The KardiaMobile 6L device was developed and is manufactured by AliveCor, an […]
AliveCor and Acutus Medical Partner to Evaluate Management and Treatment of Cardiac Arrhythmias
Collaboration Aims to Explore Benefits of Remote Monitoring of Patients Pre- and Post-Ablation MOUNTAIN VIEW, Calif., July 27, 2021 /PRNewswire/ — AliveCor, Inc. (“AliveCor”), a leading innovator in FDA-cleared personal electrocardiogram (ECG) technology and services, and Acutus Medical (“Acutus”) (Nasdaq: AFIB), an arrhythmia management company focused on improving the way cardiac arrhythmias are diagnosed and treated, today […]
FDA Clears Personal ECG Device for Measurement of QTc Interval, a Critical Marker for Patient Safety
First of its Kind FDA Action Allows In-Office and At-Home Measurement for Potentially Life-Threatening Side Effect of Certain Medications MOUNTAIN VIEW, Calif., July 8, 2021 /PRNewswire/ — AliveCor, the global leader in FDA-cleared personal electrocardiogram (ECG) technology and services, today announced it received 510(k) clearance by the U.S. Food and Drug Administration (FDA) […]