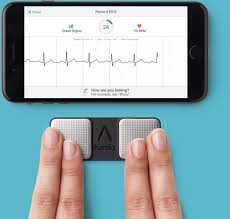
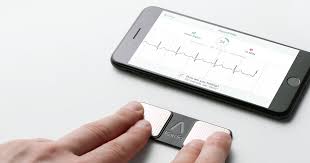
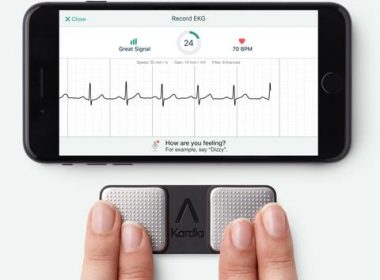
MOUNTAIN VIEW, Calif., July 24, 2019 /PRNewswire/ — AliveCor, the leader in FDA-cleared consumer electrocardiogram technology, today announced the appointment of Priya Abani as Chief Executive Officer. Abani comes to the company with decades of experience creating scale in digital platform technologies. Most recently, Abani was the General Manager for Devices and Enablement at […]
Tag: AliveCor
AliveCor and Best Buy Partner to Bring KardiaMobile to Select Stores Nationwide
MOUNTAIN VIEW, Calif., May 29, 2019 /PRNewswire/ — AliveCor, the leader in FDA-cleared consumer electrocardiogram (ECG) technology, and Best Buy Co., Inc. (NYSE: BBY) today announced a strategic partnership, bringing AliveCor’s products to select Best Buy stores across the country. This is the first time AliveCor’s products will be sold in consumer electronics stores […]
FDA Grants First Ever Clearance For Six-Lead Personal ECG Device
MOUNTAIN VIEW, Calif., May 13, 2019 /PRNewswire/ — AliveCor, the leader in FDA-cleared consumer electrocardiogram technology (ECG), today announced its third FDA clearance in three months, making KardiaMobile 6L the world’s first available six-lead personal ECG device. This highly anticipated clearance gives patients and their physicians an even more detailed view into patients’ […]
AliveCor Appoints Dr. Jacqueline Shreibati, Chief Medical Officer
MOUNTAIN VIEW, Calif., Feb. 26, 2019 /PRNewswire/ — AliveCor, the leader in FDA-cleared personal electrocardiogram (ECG) technology, today announced Jacqueline Shreibati, MD, MS, FACC, as Chief Medical Officer of the company. In her new role, Dr. Shreibati will lead AliveCor’s medical roadmap, developing innovative products that employ digital intelligence to improve heart care […]
Oxford Finance Provides $20 Million Senior Debt Facility to AliveCor, Inc.
ALEXANDRIA, Va. and MOUNTAIN VIEW, Calif., Jan. 28, 2019 /PRNewswire/ — Oxford Finance LLC (“Oxford”), a specialty finance firm that provides senior debt to life sciences and healthcare services companies, today announced the closing of a $20 million senior secured term loan to AliveCor, Inc., a privately held company focused on using artificial intelligence to improve cardiac […]
AliveCor’s Mobile ECG Technology Could Enable More Rapid Diagnosis and Treatment of Heart Attacks
MOUNTAIN VIEW, Calif., Nov. 12, 2018 /PRNewswire/ — AliveCor, the leader in FDA-cleared personal electrocardiogram (ECG) technology, has announced that a research version of its mobile technology can identify ST-elevation myocardial infarction (STEMI), the most severe form of heart attack. The findings of the ST LEUIS International Multicenter Study, to be announced at […]
FDA Designates AliveCor’s Bloodless Hyperkalemia Test a “Breakthrough Device”
MOUNTAIN VIEW, Calif., Sept. 10, 201 /PRNewswire/ — AliveCor, recently recognized as the Most Innovative Company in Artificial Intelligence, today announced that the U.S. Food and Drug Administration has given the company’s KardiaK Software Platform the rarely granted designation of “Breakthrough Device.” This designation means that the FDA will consider the technology […]
AliveCor Data Yield Reaches 25MM ECGs
MOUNTAIN VIEW, Calif., April 5, 2018 /PRNewswire/ — AliveCor, the leader in FDA-cleared personal electrocardiogram (ECG) technology, announced today that it had surpassed the milestone of 25 million recorded ECGs – by far the largest data set ever collected by any consumer ECG. The company also announced that its KardiaBand product, paired with […]
Cleveland Clinic Study Affirms Accurate Detection of Atrial Fibrillation by KardiaBand for Apple Watch
ORLANDO, Fla., March 12, 2018 /PRNewswire/ — AliveCor, the leader in FDA-cleared personal electrocardiogram (ECG) technology, today announced findings from two new studies that further support the use of its portable ECG devices to provide groundbreaking health monitoring capabilities. A new Cleveland Clinic study — which marks the first time Apple […]
AliveCor Granted Patent for Proactive Notification of Possible Heart Arrhythmias
MOUNTAIN VIEW, Calif., Dec. 12, 2017 /PRNewswire/ — AliveCor, the leader in FDA-cleared personal electrocardiogram (EKG) technology, today announced issuance of a U.S. patent which covers the use of data collected from wearable devices as a means of assisting diagnoses of heart arrhythmias, including those that may be asymptomatic. U.S. Patent […]