atHeart Medical’s reSept ASD Occluder is designed to preserve patients’ future transseptal treatment optionsBAAR, Switzerland and SANTA CLARA, Calif., June 18, 2024 /PRNewswire/ — atHeart Medical, a medical device company establishing a new standard of care for atrial septal defects (ASDs) closure, today announced promising long and short-term clinical outcomes of its reSept ASD occluder, to be presented at the medical conference CSI Frankfurt. The data from three patient cohorts show positive efficacy and safety profile that contribute to a growing body of evidence about the innovative device’s potential. reSept is the first ASD occluder with a bioresorbable, metal-free frame, designed to enable future transseptal treatment: a true evolution for patients that need transcatheter septal closure.
Continue Reading
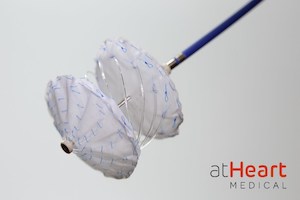
The reSept ASD Occluder has a bioresorbable, metal-free frame
A growing body of evidenceThe new reSept data to feature at CSI on June 19th include:
Positive ten-year outcomes from the first-in-human (FIH) trial showing long-term efficacy and safety of the device – to be presented by Dr. Kolja Sievert, from the CardioVascular Center Frankfurt (DE)
Promising two-year data showing complete closure in a case study from the University of Virginia (UVA) cohort of ASCENT ASD, the ongoing pivotal trial approved in the USA, Canada, France and Switzerland – discussed by Dr. Scott Lim, Professor of Medicine and Pediatrics at UVA
One-month reSept efficacy in patients with iatrogenic ASDs (iASDs), caused by mitral valve intervention, from the Canadian first-ever experience through Health Canada’s Medical Devices Special Access Program – shown by Dr. Fady Zaky from St Paul’s Hospital, Vancouver, (CA)
“Having pioneered reSept in its early days as part of ASCENT ASD in the USA, I am very pleased about the long-term outcomes from the earlier European FIH trial and the wealth of promising new data available,” said Dr. Saibal Kar, National Physician Director HCA Healthcare, USA, co-Principal Investigator of the ASCENT ASD trial. “They indicate how transcatheter ASD closure with a bioresorbable device could become an even less invasive procedure for patients long-term. This is a very exciting development for our patients.”To date around 100 patients with ASDs have been successfully treated as part of trials and special access iASD adult use in Canada, France, Germany, Switzerland and the USA. The ASCENT ASD pivotal study is currently enrolling patients in over 20 hospitals in the United States, four hospitals in France and will start treating patients in Canada and in Switzerland following Health Canada and Swissmedic’s recent authorizations.”Around 100 patients have been implanted with reSept to date. While hospitals continue to enroll patients as part of ASCENT ASD in several countries, we are excited that these positive outcomes showcase the potential for reSept to soon be the new standard of care,” said Laurent Grandidier, atHeart Medical CEO.The reSept ASD Occluder is an investigational device that aims to address the limitations of current occluders, which have metallic frames that stay in a patient’s heart for life and can limit transseptal interventions.About reSept™ ASD OccluderreSept is the first ASD occluder with a bioresorbable, metal-free frame, designed to enable future transseptal interventions. Unlike existing occluders, its frame comprises two synthetic fabric patches connected by bioresorbable filaments that overtime resorb in the body.About reSept clinical research programTo date, reSept has been implanted in around 100 patients as part of the following investigations:
First-in-Human trial in Germany – nine adult ASD patients
European registry in Switzerland and Germany – six ASD patients, of which four children
ASCENT ASD Pivotal trial – a prospective, single-arm, global multi-site clinical investigation approved by US FDA, French ANSM, Health Canada and Swissmedic; currently enrolling up to 250 patients, around 90 patients implanted to date. For more information: www.clinicaltrials.gov – Identifier: NCT04591392.
About atHeart MedicalatHeart Medical is a medical device company with offices in Switzerland and the United States committed to establish a new standard of care for treatment of atrial septal defects (ASDs). For more information: www.atheartmedical.com.About ASDCommonly described as a “hole in the heart”, an ASD is an opening in the heart septum between the left and right atria. ASDs are the second most common congenital heart defect, affecting six in 10,000 births.1 They can also be the result of procedures, such as mitral valve treatment, that require crossing the septum (iASDs). A large atrial septal defect can cause extra blood to overfill the lungs and overwork the right side of the heart. If not treated, it can lead to pulmonary hypertension, arrythmia, heart failure and increased risk of stroke.1 When ASDs require closure, the current standard of care is to implant a septal occluder with a metallic frame through a minimally invasive transcatheter procedure.SOURCE atHeart Medical