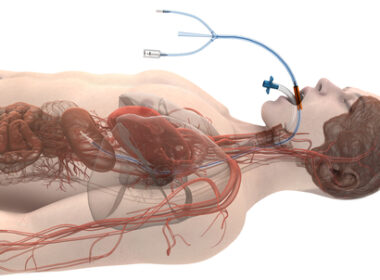
CHICAGO–(BUSINESS WIRE)–Attune Medical has been granted De Novo marketing authorization from the US Food and Drug Administration (FDA) for its ensoETM device to reduce the likelihood of ablation-related esophageal injury resulting from radiofrequency cardiac ablation procedures. “Our lab has published studies showing a 30 percent reduction in procedure time and a 14 percent […]